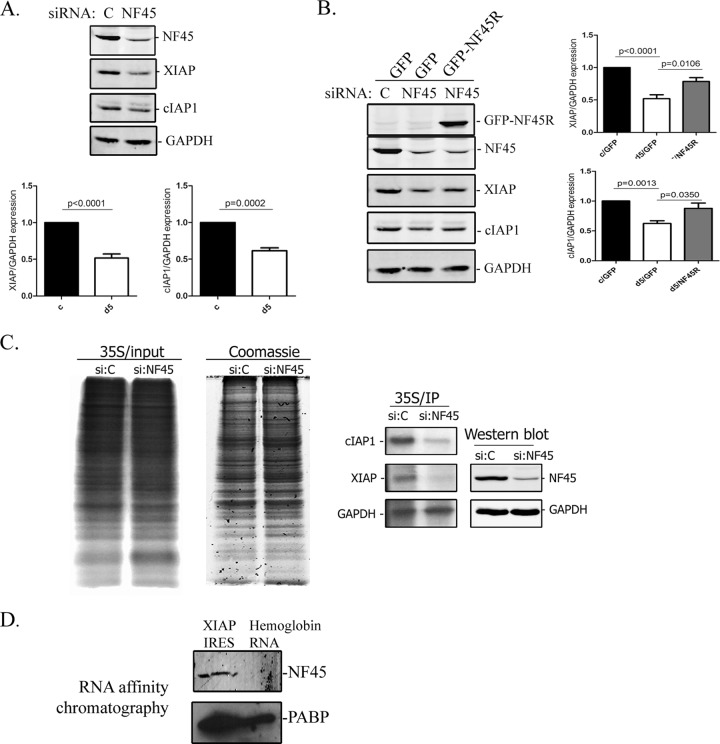
Fig 4.
NF45 regulates XIAP translation through interaction with its IRES. (A) Western blots of endogenous XIAP and cIAP1 protein expression in d5 cells relative to that in c cells. The blots and densitometry analyses are representative of at least three experiments. (B) NF45 reexpression in NF45-deficient HeLa cells rescues XIAP protein expression. The HeLa cells were transfected with 50 nM control nontargeting siRNA or NF45 siRNA (d5 siRNA) for 48 h, followed by the overexpression of GFP or GFP-NF45R for an additional 48 h. The cell extracts were analyzed by Western blotting for NF45, XIAP, cIAP1, and GAPDH expression, and protein expression was quantified. (C) De novo protein expression of cIAP1 and XIAP in NF45-deficient cells. c cells were transfected with 50 nM control or NF45 siRNA and were pulse-labeled with [35S]methionine. 35S-labeled and Coomassie blue-stained total protein, as well as specific cIAP1, XIAP, and GAPDH immunoprecipitates, are shown. (D) NF45 interacts specifically with the XIAP IRES. NF45 and PABP Western blotting of an RNA affinity chromatography preparation was performed using the XIAP IRES or hemoglobin RNA.