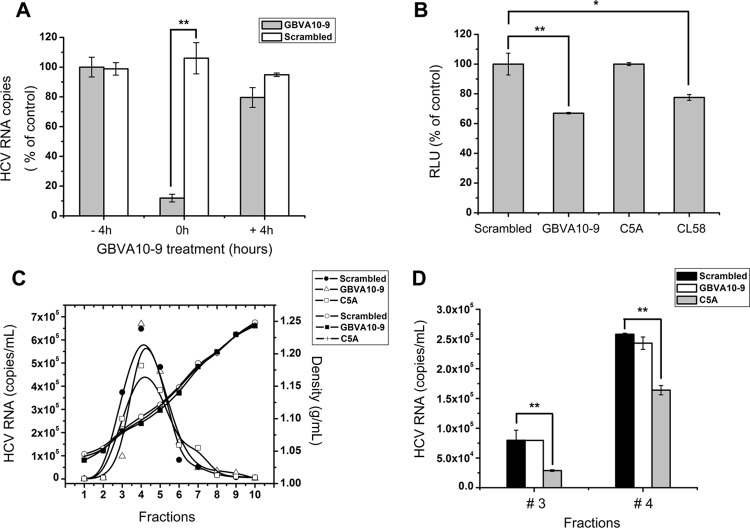
Fig 3.
Antiviral mechanism of GBVA10-9. (A) GBVA10-9 (2 μM) and scrambled peptide (2 μM) were added to Huh7.5.1 cells 4 h prior to (− 4h) or after (+ 4h) inoculation of HCVcc (multiplicity of infection = 0.1) or together with the virus (0h). For the − 4h cells, peptides were washed away before virus inoculation, whereas the 0h and + 4h cells were incubated with peptides along with the virus for a total of 12 h before the medium was changed. At 72 h postinfection, the intracellular HCV RNA was quantified by qRT-PCR. (B) Fusion inhibition assay. 293T cells expressing Stop-Luc and HCV receptor CLDN1 (recipient cells) were mixed at a 1:1 ratio with donor cells expressing Cre and HCV E1E2 (H77, genotype 1a) to initiate cell-cell fusion. The indicated peptides were added, and luciferase activity was measured 24 h thereafter. (C) Concentrated HCVcc was equally divided into PBS containing GBVA10-9 (2 μM), C5A (18 μM), or scrambled peptide (2 μM), incubated at 37°C for 2 h, and then analyzed by velocity sedimentation ultracentrifugation. After weighing, the sucrose gradient fractions were collected for RNA extraction. The amount of HCV RNA was quantified by qRT-PCR and plotted on the left y axis, while the density of each fraction was plotted on the right y axis. (D) The virus-containing sucrose gradients of fraction 3 and 4 as described for panel C were transferred to naïve Huh7.5.1 cells, and the infectivity titer of each fraction was evaluated 48 h thereafter.