Abstract
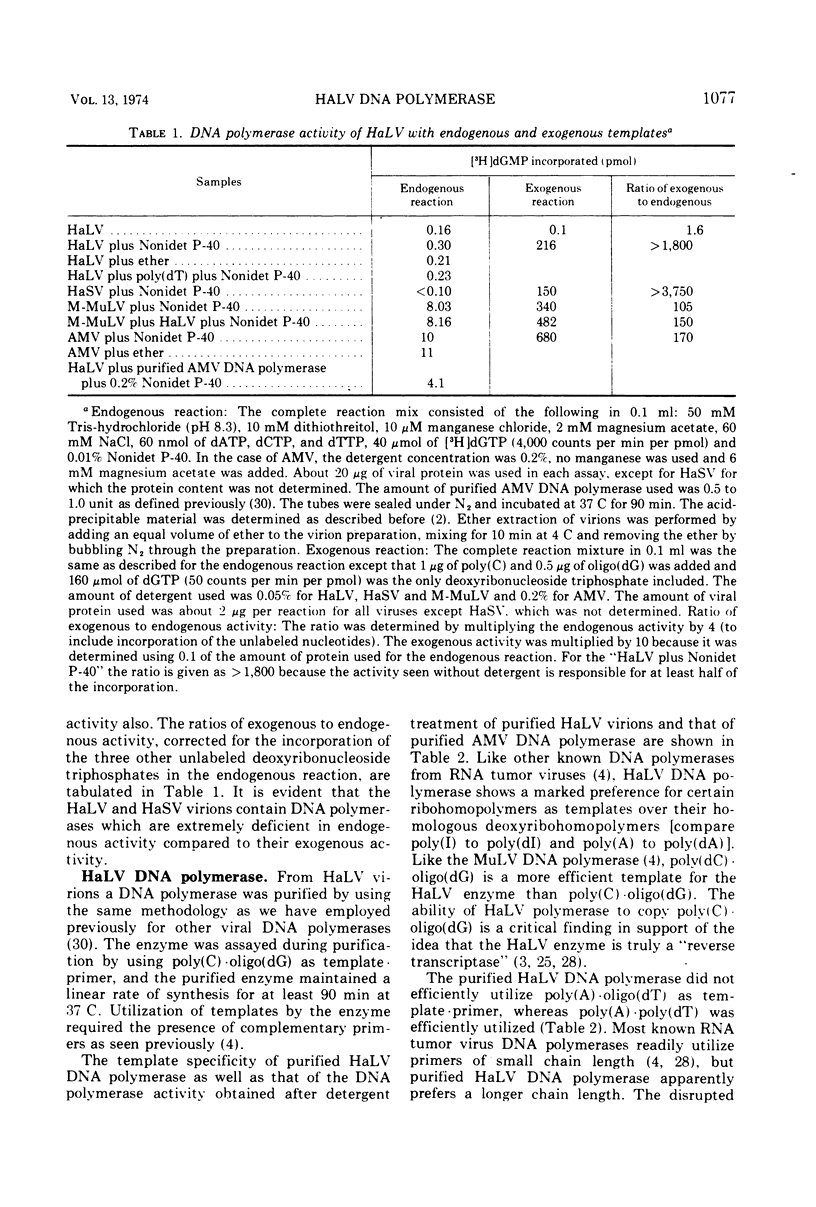
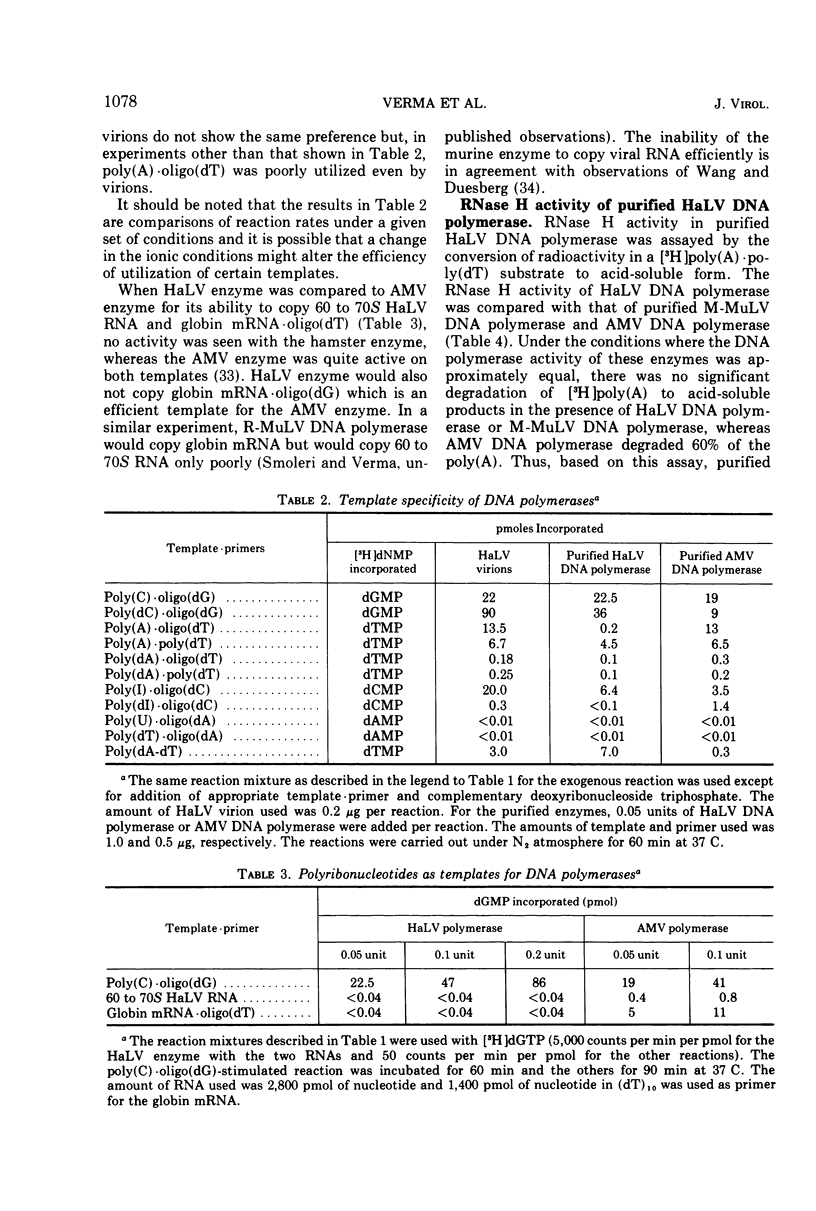
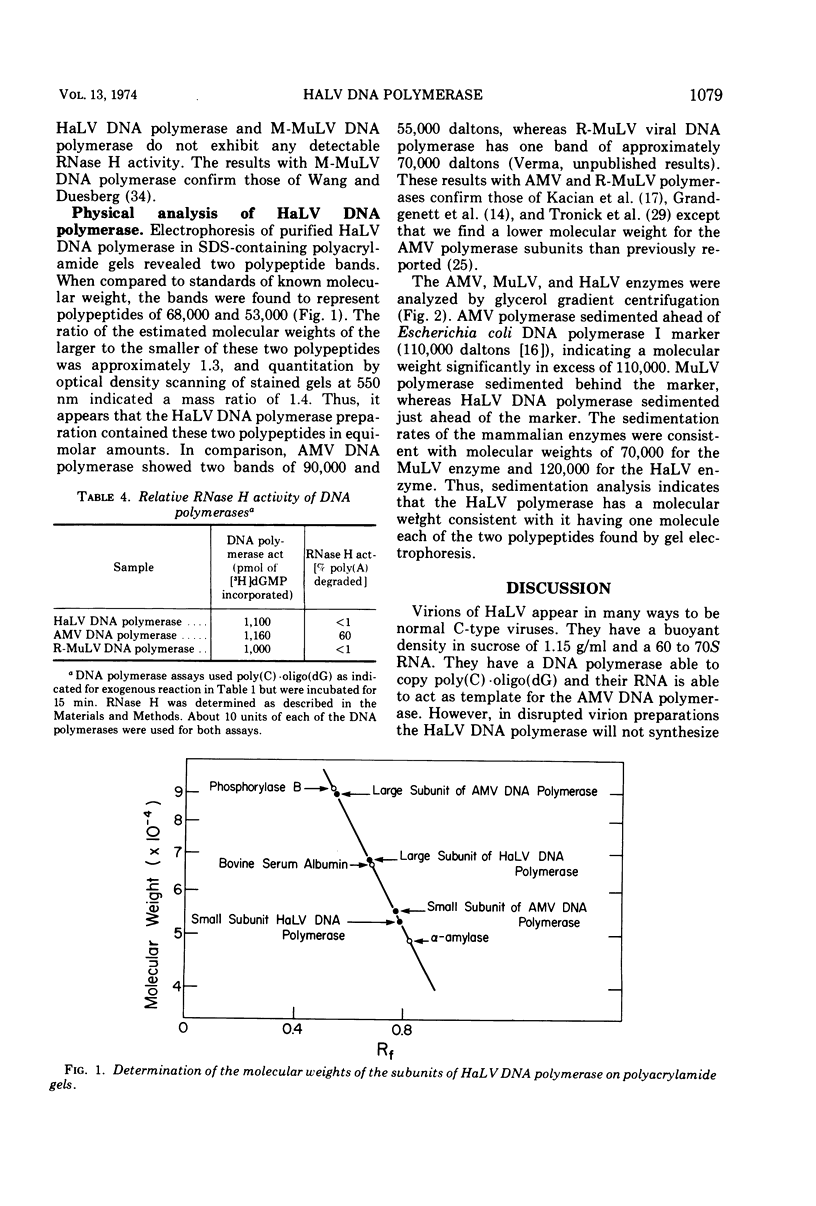
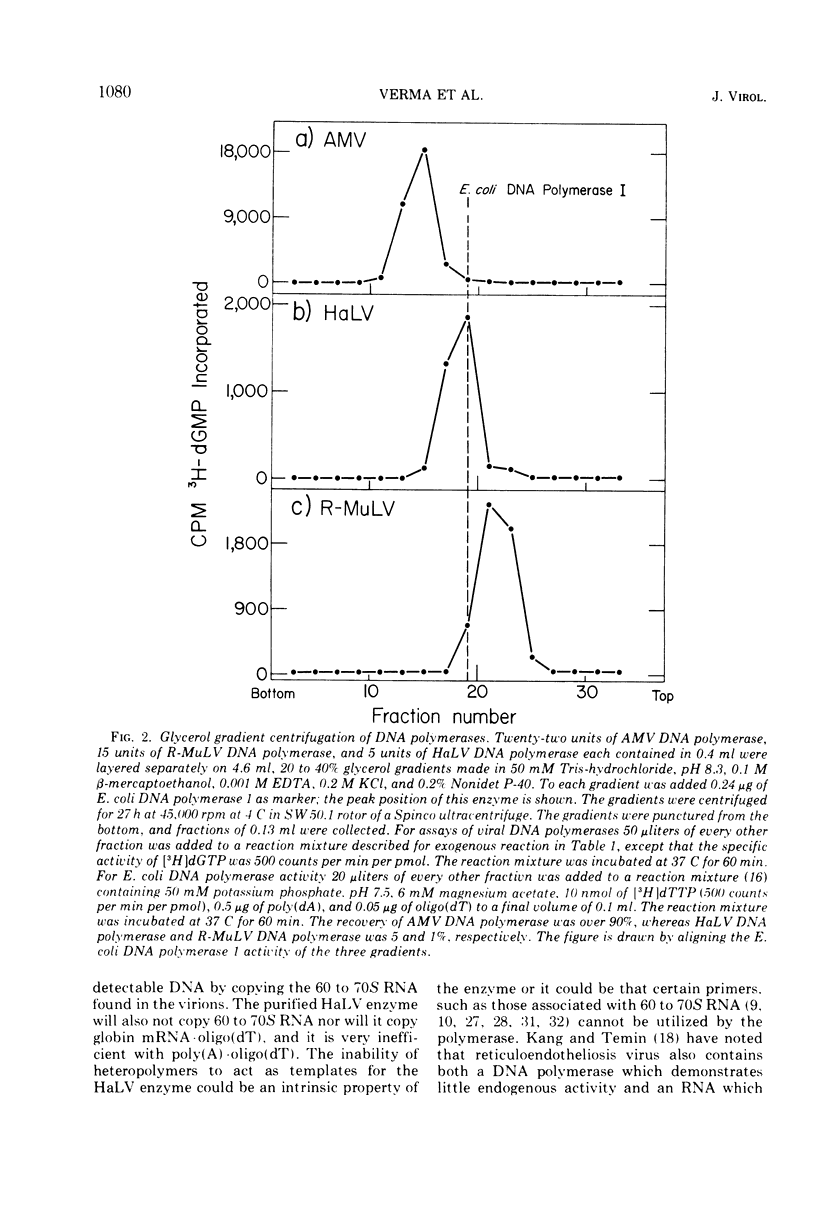
Infectious hamster leukemia virus (HaLV) contains a DNA polymerase different from those of murine and avian viruses. No endogenous reaction directed by the 60 to 70S RNA of HaLV could be demonstrated in detergenttreated HaLV virions, nor could the purified DNA polymerase copy added viral RNA. The virion RNA could, however, act as template for added avian myeloblastosis virus DNA polymerase and the HaLV DNA polymerase could efficiently utilize homopolymers as templates. The HaLV enzyme was like other reverse transcriptases in that certain ribohomopolymers were much better templates than the homologous deoxyribohomopolymers. No ribonuclease H activity could be shown in the HaLV enzyme, but neither could activity be found in the murine leukemia virus DNA polymerase. The hamster enzyme was unique in that poly(A) ·oligo(dT) was a poor template, and globin mRNA primed with oligo(dT) was totally inactive as a template. Its uniqueness was also indicated by its subunit composition; electrophoresis of the HaLV DNA polymerase in sodium dodecyl sulfate-containing polyacrylamide gels revealed equimolar amounts of two polypeptides of molecular weight 68,000 and 53,000. The sedimentation rate of the enzyme in glycerol gradients was consistent with a structure containing one each of the two polypeptides. The enzyme thus appears to be structurally distinct from other known virion DNA polymerases. Its inability to carry out an endogenous reaction in vitro might result from an inability to utilize certain primers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Simons P. J., Chesterman F. C., Harvey J. J. Murine sarcoma virus (Harvey): characteristics of focus formation in mouse embryo cell cultures, and virus production by hamster tumor cells. Int J Cancer. 1968 Mar 15;3(2):265–272. doi: 10.1002/ijc.2910030212. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Flügel R. M., Rapp U., Wells R. D. RNA-DNA covalent bonds between the RNA primers and the DNA products formed by RNA tumor virus DNA polymerase. J Virol. 1973 Dec;12(6):1491–1502. doi: 10.1128/jvi.12.6.1491-1502.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Kelloff G. J., Gilden R. V., Lane W. T., Swain A. P., Huebner R. J. Activation and isolation of hamster-specific C-type RNA viruses from tumors induced by cell cultures transformed by chemical carcinogens. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2386–2390. doi: 10.1073/pnas.68.10.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Russell E., Sarma P. S., Sarin P. S., Hall W., Chopra H. C. Properties of noninfectious and transforming viruses released by Murine sarcoma virus-induced hamster tumor cells. J Virol. 1973 Oct;12(4):931–936. doi: 10.1128/jvi.12.4.931-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffi A., Schramm T., Bender E., Graffi I., Horn K. H., Bierwolf D. Cell-free transmissible leukoses in Syrian hamsters, probably of viral aetiology. Br J Cancer. 1968 Sep;22(3):577–581. doi: 10.1038/bjc.1968.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. DNA polymerase activity associated with RNA tumor viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):143–147. doi: 10.1073/pnas.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Chang N. H., Lee Y. K., Gilden R. V. Envelope antigen relationships among three hamster-specific sarcoma viruses and a hamster-specific helper virus. J Gen Virol. 1970 Oct;9(1):19–33. doi: 10.1099/0022-1317-9-1-19. [DOI] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Lee Y. K., Toni R., Gilden R. Hamster-tropic sarcomagenic and nonsarcomagenic viruses derived from hamster tumors induced by the Gross pseudotype of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1970 Feb;65(2):310–317. doi: 10.1073/pnas.65.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Oroszlan S., Toni R., Gilden R. V. Immunological identity of the group-specific antigen of hamster-specific C-type viruses and an indigenous hamster virus. J Gen Virol. 1970 Oct;9(1):27–33. doi: 10.1099/0022-1317-9-1-27. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., O'Donnell P. V., Sanders F. K. Serological identification of hamster oncornaviruses. Nat New Biol. 1971 Apr 28;230(17):282–284. doi: 10.1038/newbio230282a0. [DOI] [PubMed] [Google Scholar]
- Stenback W. A., Van Hoosier G. L., Jr, Trentin J. J. Virus particles in hamster tumors as revealed by electron microscopy. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1219–1223. doi: 10.3181/00379727-122-31365. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Scolnick E. M., Parks W. P. Reversible inactivation of the deoxyribonucleic acid polymerase of Rauscher leukemia virus. J Virol. 1972 Oct;10(4):885–888. doi: 10.1128/jvi.10.4.885-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Baltimore D. Purification of the RNA-directed DNA polymerase from avian myeloblastosis virus and its assay with polynucleotide templates. Methods Enzymol. 1974;29:125–130. doi: 10.1016/0076-6879(74)29015-3. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H. DNA polymerase of murine sarcoma-leukemia virus: lack of detectable RNase H and low activity with viral RNA and natural DNA templates. J Virol. 1973 Dec;12(6):1512–1521. doi: 10.1128/jvi.12.6.1512-1521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavada J., Macpherson I. Transformation of hamster cell lines in vitro by a hamster sarcoma virus. Nature. 1970 Jan 3;225(5227):24–26. doi: 10.1038/225024a0. [DOI] [PubMed] [Google Scholar]



