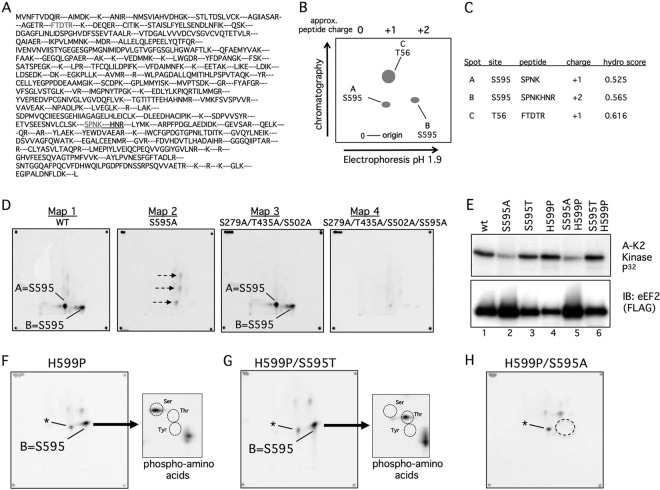
Fig 2.
Phosphopeptide and phosphoamino acid analyses of eEF2 phosphorylation by cyclin A-CDK2 in vitro. (A) Predicted eEF2 tryptic peptides. The S595 and T56 peptides are in grey, and the extended peptide caused by H599P is underlined. (B) Schematic of phosphopeptide mapping procedure. Trypsinized peptides were run on TLC plates in two dimensions. The first was electrophoretic separation based on charge, and the second was chromatographic separation based on hydrophobicity. The positions of the three major peptides discussed in the paper are shown. (C) Calculated charges and hydrophobicity of the three major phosphopeptides. (D) Two-dimensional phosphopeptide maps of WT eEF2 (map 1) and the indicated eEF2 mutants (maps 2 to 4) after in vitro phosphorylation by cyclin A-CDK2. The corner spots on each map are orientation markers. The two S595 peptides are indicated (spots A and B). Three minor phosphorylation sites (map 2, arrows) that are eliminated by the 279/435/502 alanine mutations (maps 3 and 4) are also indicated. (E) A S595-to-threonine (S595T) mutation is normally phosphorylated. The two S595A mutants were overloaded to reveal the residual phosphorylations shown in panel H. Note the reduced amount of phosphorylation of S595A and S595A/H599P despite their overexpression relative to the other eEF2 proteins. A-K2 Kinase, cyclin A-CDK2. (F) Phosphopeptide map of eEF2 H599P. Phospho-S595 shifts to a single phosphopeptide (spot B), and phosphoamino analysis of spot B reveals only phosphoserine. Circles indicate the positions of marker phosphoamino acids, and the asterisk denotes a minor phosphopeptide that comigrates near spot A. (G) Analyses similar to those shown in panel F show that the S595T mutant converts spot B from phosphoserine to phosphothreonine. (H) Mapping of residual phosphorylations of eEF2 S595A/H599P by cyclin A-CDK2. The dotted circle indicates loss of spot B.