Fig 7.
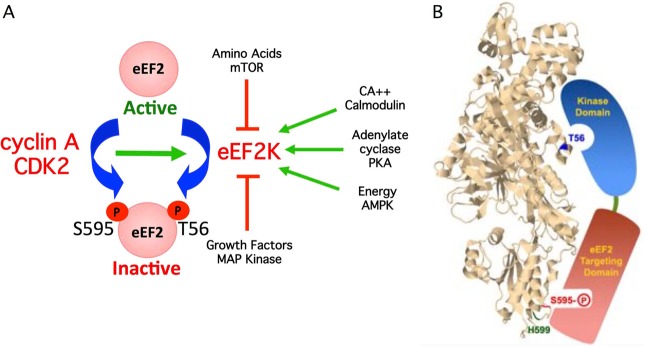
(A) Model depicting how S595 phosphorylation can globally regulate eEF2 activity by provoking T56 phosphorylation by eEF2K. Pathways that activate eEF2K are shown as green arrows, and pathways that inhibit eEF2K are shown in red. Increased S595 phosphorylation by cyclin A-CDK2 enhances T56 phosphorylation and eEF2 inhibition when eEF2K is activated. (B) Model depicting hypothesized interaction of the two functional eEF2K domains with eEF2 S595 and T56. The positions of T56, S595, and H599 were superimposed on the structure of budding yeast eEF2. The image is from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB; www.pdb.org) of PDB ID 2P8W (29). Residues were localized with Jmol, an open-source Java viewer for chemical structures in 3D (http://www.jmol.org/).
