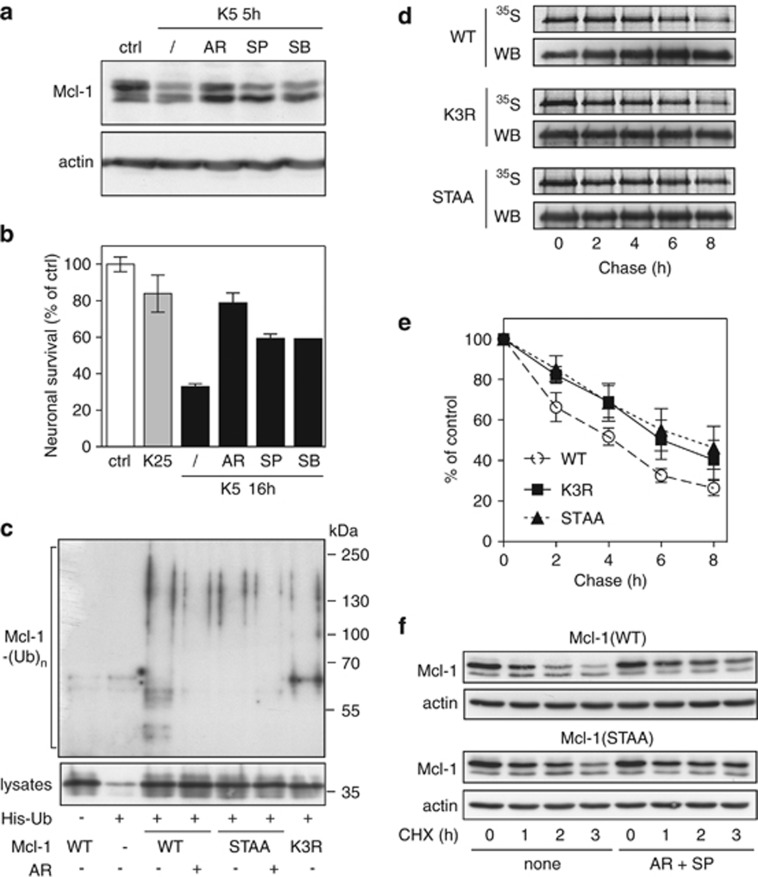
Figure 3.
Prior phosphorylation of Mcl-1 is required for its ubiquitination and subsequent degradation. (a) CGNs were left untreated (ctrl) or washed and switched to K5 medium in the presence or absence of 10 μM AR-A014418, 10 μM SP600125 or 20 μM SB 203580 for 5 h. Total protein extracts were analyzed by immunoblotting using antibodies against Mcl-1 and actin. (b) CGN cultures were incubated in K25 or K5 medium in the presence or absence of 10 μM AR-A014418, 10 μM SP600125 or 20 μM SB 203580 for 16 h. Then, neuronal viability was determined by MTT assay. Data are the means±S.D. of triplicate determinations obtained in a typical experiment representative of three independent experiments. Results are expressed as the percentage of surviving neurons compared to neurons maintained in initial culture medium (ctrl). (c) CGNs were transfected with untagged Mcl-1(WT), Mcl-1(STAA) or Mcl-1(K3R), or with empty plasmid (−), together with His-tagged ubiquitin (+) or empty plasmid (−) for 18 h. Then, neurons were incubated for 6 h with 25 μM MG-132, in the presence or the absence of 10 μM AR-A011418 (AR). The ubiquitinated proteins were purified using nickel beads and analyzed by western blotting using anti-Mcl-1 antibody to detect ubiquitin-conjugated Mcl-1. In a separate SDS-PAGE, samples of the input lysates used for the purification were analyzed with anti-Mcl-1 antibody to estimate the expression level of the different forms of transfected Mcl-1. (d) CGNs were transfected with Mcl-1(WT)-GFP, Mcl-1(K3R)-GFP or Mcl-1(STAA)-GFP for 18 h. Then, neurons were metabolically labeled with [35S]-Met for 2 h (pulse) and harvested at different times after washing and incubation in K5 medium (chase). The different forms of Mcl-1-GFP were then immunoprecipitated using GFP-trap beads, separated by SDS-PAGE and visualized by both autoradiography (35S) and immunodetection of Mcl-1 (WB). Note that a higher amount of Mcl-1(WT) was immunoprecipitated compared to the mutants in the three last time points. This may give the feeling that it is more stable than it actually is. (e) The intensity of the bands on the autoradiograms and on the immunoblots of different experiments performed as in (d) was quantified. For each experiment, the radioactivity associated with the different forms of Mcl-1-GFP was normalized by the amount of protein immunoprecipitated in each condition and plotted against chase time. Data are the mean±S.E.M. of three independent experiments. (f) CGNs were transfected with untagged Mcl-1(WT) or Mcl-1(STAA). Eighteen hours after transfection, neurons were pre-incubated for 1 h in K5 medium in the presence or the absence of 10 μM AR-A011418 and 10 μM SP600125 (AR+SP). Then, 10 μg/ml cycloheximide (CHX) was added, and neurons were harvested after the indicated times after CHX addition. Proteins were analyzed by western blot using antibodies against Mcl-1 and actin