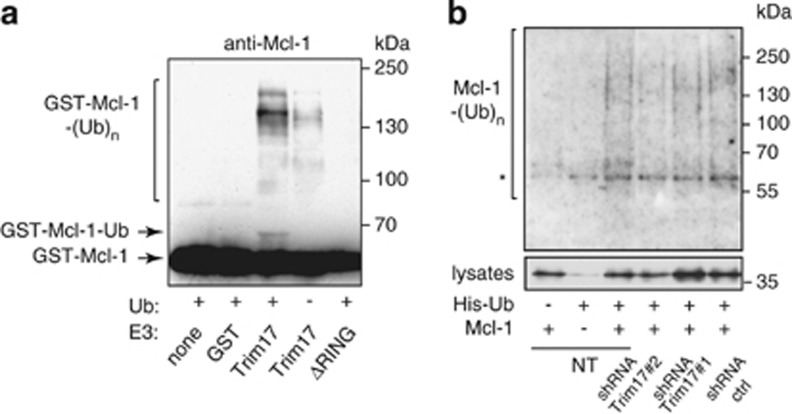
Figure 8.
Trim17 promotes Mcl-1 ubiquitination both in vitro and in vivo. (a) Purified recombinant mouse Mcl-1 was first phosphorylated by recombinant JNK and GSK3 in vitro. Then, it was incubated for 2 h in the in vitro ubiquitination reaction mix with different recombinant proteins: GST, GST-Trim17(WT) or GST-Trim17(ΔRING), in the presence or the absence of ubiquitin, as indicated. Mcl-1 ubiquitination was examined by anti-Mcl-1 immunoblotting. The arrows indicate unmodified Mcl-1 (GST-Mcl-1) and mono- or di-ubiquitinated form of Mcl-1 (GST-Mcl-1-Ub). Higher bands are poly- or multi-ubiquitinated forms of Mcl-1 (GST-Mcl-1-(Ub)n). (b) CGNs were left untreated (non transduced: NT) or were transduced with lentiviral particles expressing shRNA sequences (one control and two against Trim17), one day after plating. At DIV 5, neurons were transfected with Mcl-1(WT), His-tagged ubiquitin or both for 18 h. The ubiquitinated proteins were purified using nickel beads, separated by SDS-PAGE and visualized by immunoblotting with anti-Mcl-1 antibody to detect ubiquitin-conjugated Mcl-1. In a separate SDS-PAGE, samples of the input lysates were analyzed with anti-Mcl-1 antibody to estimate the expression level of transfected Mcl-1 in the different conditions. Normalization of the ubiquitination signal by the expression level of Mcl-1 in lysates shows that ubiquitination is approximately reduced by half after transduction with both shRNA-Trim17#1 and #2 compared to shRNA ctrl. *Indicates a non-specific band