Figure 5.
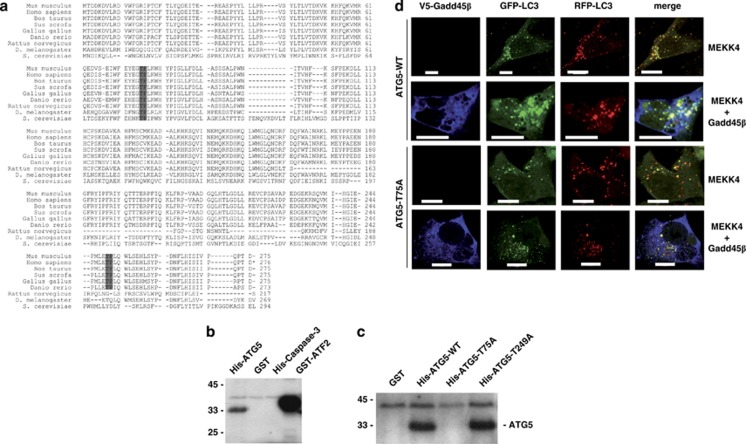
Atg5 is a substrate for p38. (a) The protein sequences of Atg5 proteins from different species were analyzed for potential phosphorylation sites of p38 (XS/TPX consensus). Two putative phosphorylation sites could be identified at threonine 75 and threonine 249 of murine Atg5. (b) The indicated proteins were tested for phosphorylation by p38 in an in vitro kinase assay. His-Caspase-3 and GST served as a negative control, whereas GST-ATF2 was used as a positive control. (c) In vitro kinase assay with WT His-Atg5 as well as with His-Atg5, in which T75 and T249 had been mutated to alanine, respectively. GST served as a negative control. (d) Atg5−/− MEF cells stably reconstituted with WT or a T75A mutant of Atg5 were transfected with MEKK4 and an RFP-GFP-LC3 reporter either with or without Gadd45β co-expression. At 24 h post transfection, cells were starved for 4 h in HBSS. Subsequently, cells were fixed, stained for Gadd45β expression and analyzed by confocal microscopy for RFP-GFP-LC3 localization. Bars, 10 μm