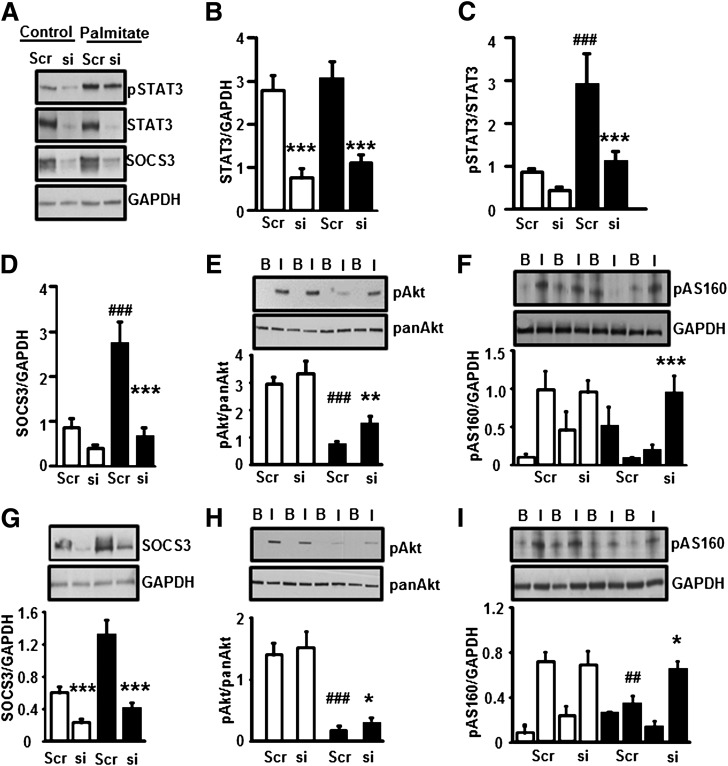
FIG. 5.
Effect of STAT3 silencing on protein phosphorylation. Myotubes were transfected with siRNA against a scrambled sequence or STAT3. Transfected myotubes were incubated in the absence (control) or presence of palmitate (0.25 mmol/L) for 24 h. A: Representative immunoblots show the effect of palmitate and STAT3-specific siRNA on p-STAT3, STAT3, SOCS3, and GAPDH protein abundance. B: STAT3 was reduced 70% by siRNA STAT3 silencing. Phosphorylation of STAT3 (C) and expression of SOCS3 proteins (D) were measured. p-Akt (E) and p-AS160 (F) were measured in myotubes after incubation in the absence or presence of insulin (60 nmol/L). □, control myotubes; ■, palmitate-treated myotubes. ***P < 0.001, **P < 0.01 vs. siRNA; ###P < 0.001 vs. control scrambled, n = 6. Myotubes were transfected with siRNA against a scrambled sequence or SOCS3. Transfected myotubes were incubated in the absence (control) or presence of palmitate (0.25 mmol/L) for 24-h. G: SOCS3 was reduced 75% by siRNA SOCS3 silencing. Phosphorylation of p-Akt (H) and p-AS160 (I) was measured in myotubes after 20 min incubation in the absence or presence of insulin (60 nmol/L). □, control myotubes; ■, palmitate-treated myotubes. ***P < 0.001, *P < 0.05 vs. siRNA; ###P < 0.001, ##P < 0.01 vs. control scrambled, n = 6.