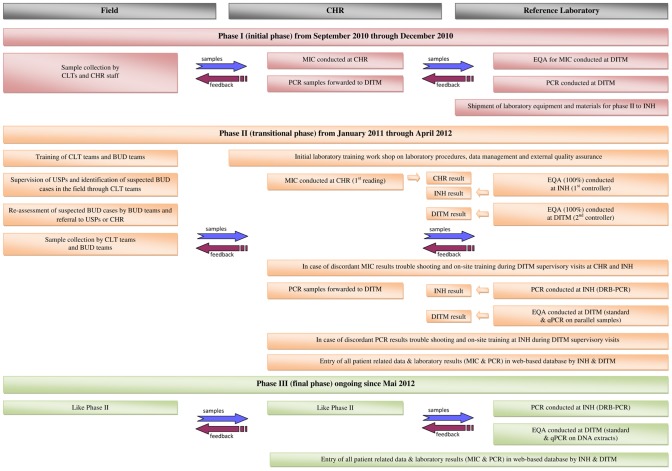
Figure 1. Stepwise approach to implementation of diagnostic laboratory facilities at INH.
Figure 1 describes the process of implementation of diagnostic laboratory facilities at INH in three phases and the flow of samples as well as the flow of feedback between the Department for Infectious Diseases and Tropical Medicine (DITM), Ludwig-Maximilians-University, Munich, Germany, the “Institut National d'Hygiène” (INH), Lomé, Togo, the “Centre Hospitalier Régional Maritime” (CHR), Tsévié, Togo, and field staff. BUD, Buruli ulcer disease; CLT, “Contrôleur Lèpre-TB-Buruli” – district controllers; DRB-PCR, dry-reagent-based IS2404 PCR; EQA, external quality assurance; MIC, microscopic detection of acid fast bacilli by Ziehl-Neelsen staining; PCR, polymerase chain reaction; qPCR, IS2404 quantitative real-time PCR; standard PCR, conventional gel-based IS2404 PCR; USP, “Unité de Soins Périphérique” – peripheral health posts.