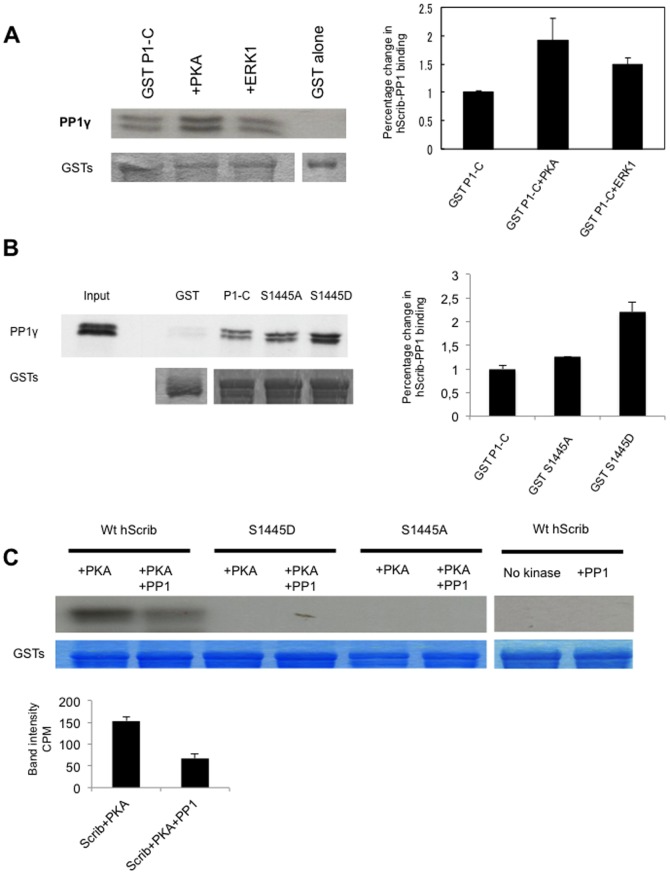
Figure 3. hScrib is a substrate of PP1γ.
A) Purified GST-hScrib fusion protein was in vitro phosphorylated with purified PKA or ERK1 as described previously (19) and then incubated with PP1γ for 20 mins at 30°C. Bound PP1γwas detected by western blotting with anti PP1γ antibody. The lower panel shows the ponceau stain of the nitrocellulose, and the upper right panel shows the quantitations from three independent experiments. Note that hScrib phosphorylated by PKA exhibits increased association with PP1γ. B) Purified PP1γ was incubated with purified full length wild type GST-hScrib fusion protein (P1-C), the mutants S1445A, S1445D or GST alone as a control. After extensive washing the bound PP1γ was ascertained by western blotting. The upper panel shows the result of the western blot, with the 20% input of PP1γ also shown for comparison. The lower panel shows the ponceau stain of the nitrocellulose. The histogram shows the quantitation from three independent experiments. C) Purified GST-hScrib wild type and PKA phospho-site mutants of hScrib were in vitro phosphorylated with purified PKA in the presence of radiolabeled ATP as described previously (19) and incubated with PP1γ for 20 mins at 30°C. The remaining level of phosphorylated hScrib was then determined following SDS PAGE and autoradiography. The two right-hand lanes show lack of phosphorylation of hScrib in the absence of PKA, whilst the lower panels show the Coomassie stain of the gel demonstrating equal levels of the GST-hScrib fusion protein throughout. The quantitation of hScrib phosphorylation from three independent experiments is also shown.