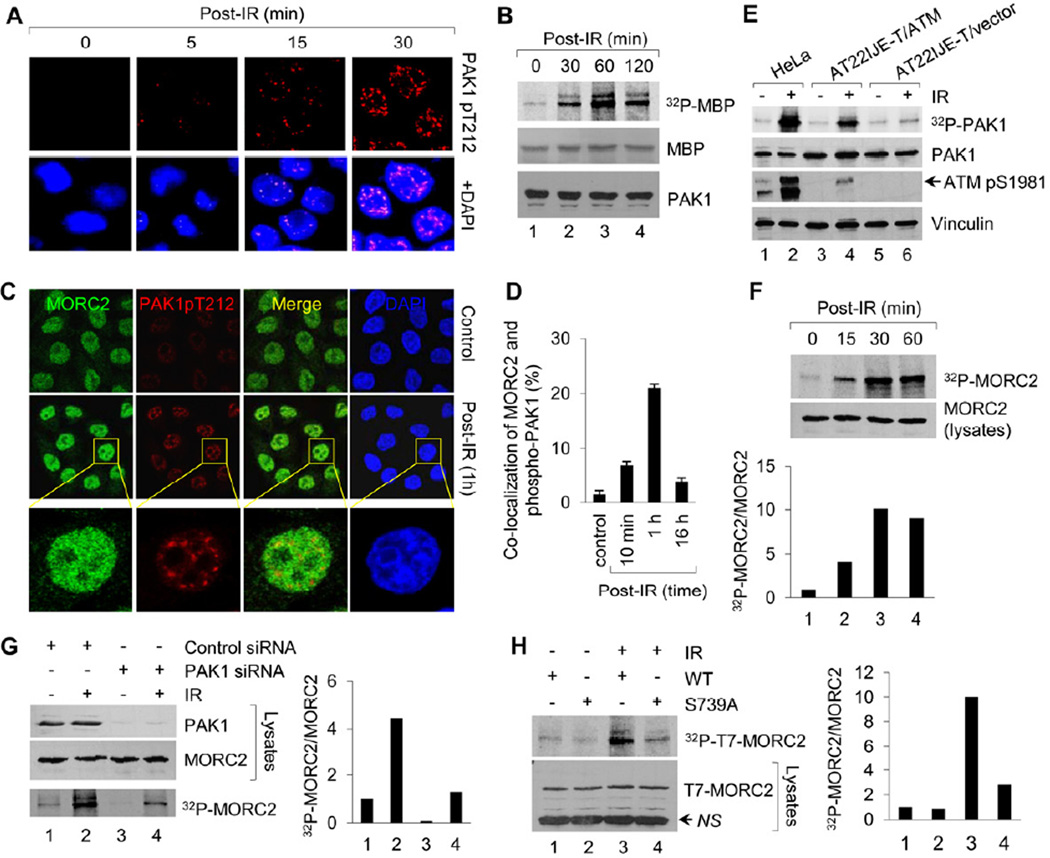
Figure 2. IR Induces MORC2 Phosphorylation in a PAK1-dependent Manner.
(A) HeLa cells were untreated or treated with 10 Gy of IR, harvested at the indicated time points for immunofluorescence staining with the indicated antibodies. Nuclei were visualized by DAPI staining (blue). (B) HeLa cells were untreated or treated with 10 Gy of IR and harvested at the indicating time points. PAK1 immunocomplex was subjected to in vitro kinase assay in the presence of [32P]ATP and MBP as substrates. (C-D) HeLa cells were treated with or without IR and harvested at the indicated time points for immunofluorescence staining of MORC2 and phospho-PAK1 (Thr212). The representative images (C) and quantitation results of co-localization of MORC2 with phospho-PAK1 (D) are shown. (E) Cells were radiolabeled with [32P] orthophosphate overnight. After 1 h of IR treatment, protein extracts were immunoprecipitated with an anti-PAK1antibody, separated by SDS-PAGE, and analyzed autoradiography. Total lysates were immunoblotted with the indicated antibodies for internal controls. (F) HeLa cells were metabolically labeled with [32P] orthophosphoric acid overnight and treated with or without IR. Nuclear extracts were immunoprecipitated with an anti-MORC2 antibody and analyzed by autoradiography. Total lysates were immunoblotted with anti-MORC2 antibody for internal control. (G) HeLa cells were transfected with control siRNA or PAK1 siRNA, metabolically labeled with [32P] orthophosphoric acid overnight. After 1 h of IR treatment, nuclear extracts were subjected to in vivo MORC2 phosphorylation assay. Total lysates were immunoblotted with anti-PAK1 and anti-MORC2 antibodies for internal controls. (H) MCF-7 cells stably expressing wild-type or MORC2-S739A mutant were labeled with [32P]-orthophosphoric acid overnight and treated with or without IR. After 1 h of IR treatment, nuclear extract were immunoprecipitated with anti-T7 agarose beads and subjected to in vivo phosphorylation assay. In F-H, the band densities were quantified using ImageJ software and the results were normalized to the signal for lane 1. NS, non-specific.