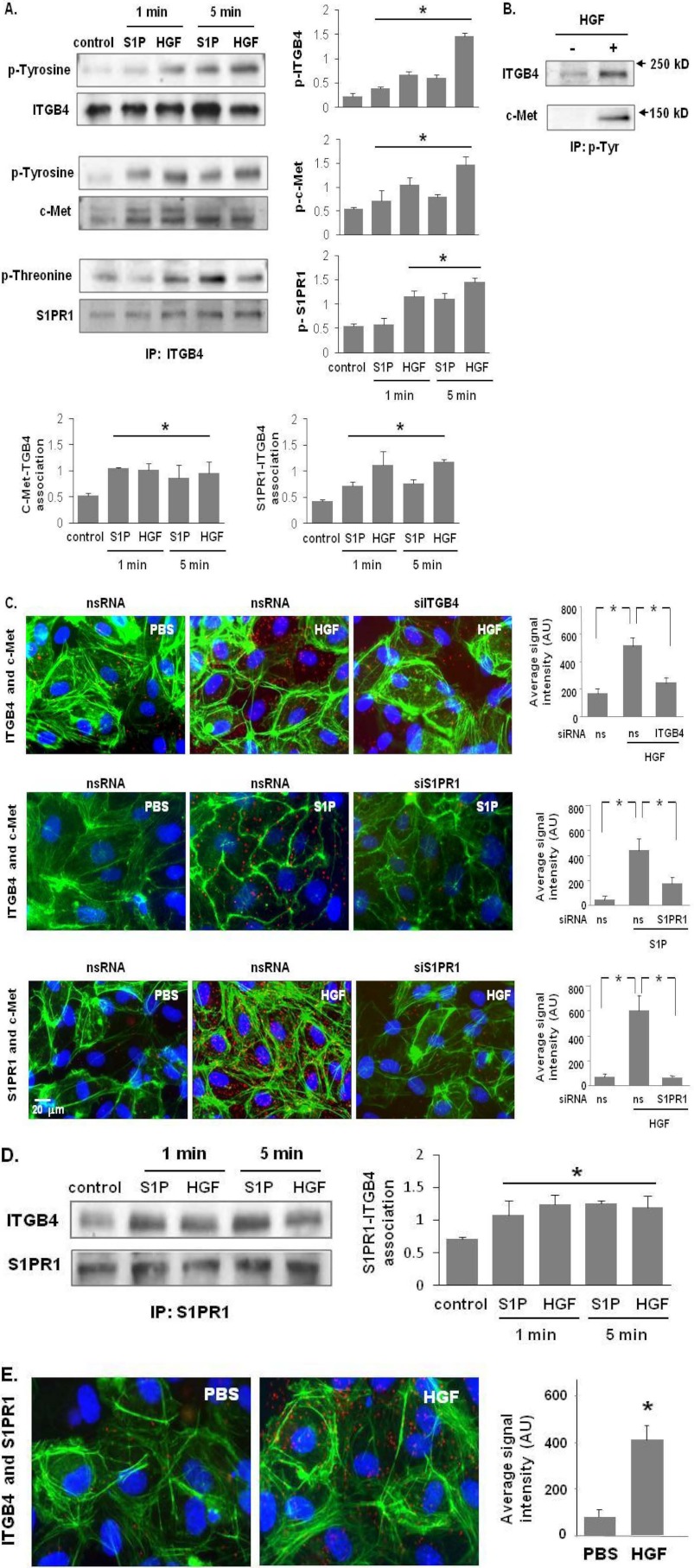
FIGURE 2.
c-Met/S1PR1/ITGB4 complex modulation by HGF and S1P. A, HPAEC were treated with either S1P (1 μm) or HGF (25 ng/ml) for 1 and 5 min. Cell lysates were subjected to immunoprecipitation with an anti-ITGB4 antibody and tyrosine or threonine phosphorylation corresponding to the same molecular weight was determined via repeat immunoblotting on the same membrane (representative blots shown, densitometric data expressed as fold change phosphorylation relative to control and normalized to total protein, n = 3/group, *, p < 0.05 compared with untreated contols). B, in separate experiments, HPAEC were treated with HGF (25 ng/ml, 5 min) and immunoprecipitation performed with an antibody specific for tyrosine phosphorylation prior to immunoblotting for c-Met and ITGB4 (representative blots shown). C, associations of c-Met with ITGB4 induced by HGF (upper panels) and S1PR1 (middle panels), as well as HGF-induced associations between c-Met and S1PR1 were detected by in situ proximity ligation assay (red dots) in untreated HLMVEC as well as both cells transfected with specific siRNA (ITGB4 or S1PR1) and control cells (ns siRNA) prior to treatment with HGF (25 ng/ml, 5 min) or S1P (1 μm, 5 min). Average signal intensity for each condition was quantified as described under “Experimental Procedures” (n = 4/condition, *, p < 0.05). D, HPAEC were treated with either S1P (1 μm) or HGF (25 ng/ml) and lysates then immunoprecipitated with an anti-S1PR1 antibody followed by immunoblotting with an anti-ITGB4 or anti-S1PR1 antibody. Representative blots and densitometry of the association between S1PR1 and ITGB4 are shown. (n = 3/condition, *, p < 0.05 compared with untreated controls). E, interaction of S1PR1 and ITGB4 in response to HGF treatment (25 ng/ml, 5 min) was also as assessed by PLA in situ.