FIGURE 3.
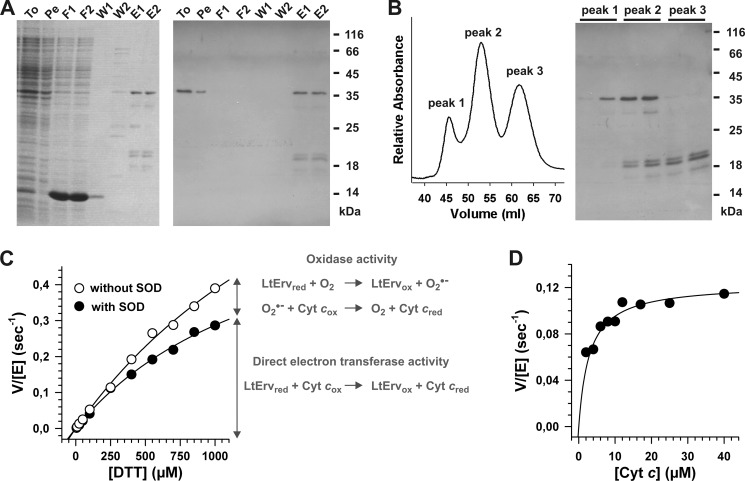
Two step purification and enzymatic activity of recombinant His-tagged LtErv. A, LtErv was first purified by Ni-NTA chromatography. Protein-containing fractions were analyzed by reducing SDS-PAGE (15% gel, left side) and Western blotting against the His tag (right side). Recombinant LtErv has a theoretical molecular mass of 35.9 kDa. To, total cells; Pe, cell pellet after first sonication; F1/2, flow through 1 and 2; W1/2, wash 1 and 2; E1/2, eluate 1 and 2. B, eluates from panel A were further purified by gel filtration chromatography resulting in three different peaks. Peak 1 contained pure full-length LtErv. Depending on the protein preparation, an additional band of ∼30 kDa was visible in peak 2. Western blotting against the N-terminal His tag revealed that this protein species lacks the presumably flexible C-terminal arm of the KISS domain (see architecture model in Fig. 1C). Peaks 2 and 3 contained three C-terminally truncated LtErv species consisting of the conserved Erv/ALR domain (17.1 kDa) with shorter and longer fragments of the KISS domain. C, the sulfhydryl:cytochrome c oxidoreductase activity of purified LtErv was analyzed with DTT and 40 μm cytochrome c in the presence or absence of SOD. In the presence of SOD the specific electron transferase activity was measured, whereas an additional unspecific oxidase activity resulted in a higher activity in the absence of SOD. Data points were averaged from two independent protein purifications. D, shown is a variation of the cytochrome c concentration revealing Michaelis-Menten kinetics with an apparent Km value around 3 μm. Measurements were performed with 0.1 mm DTT in the presence of SOD.