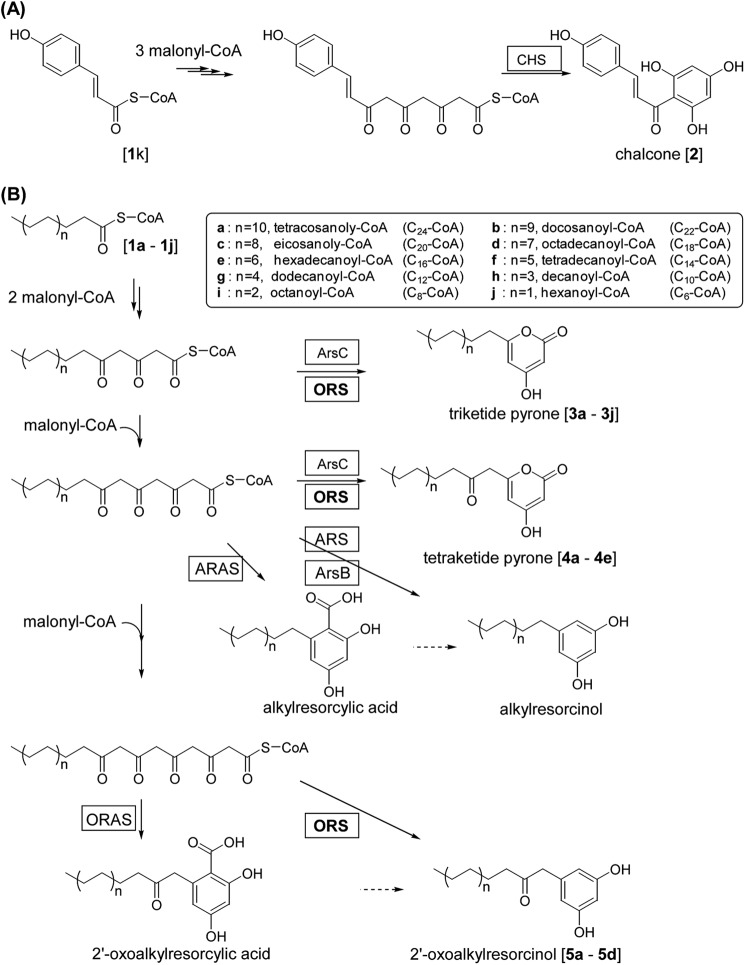
FIGURE 1.
Reactions catalyzed by type III PKSs. A, CHS condenses a p-coumaroyl-CoA with three molecules of malonyl-CoA and cyclizes the tetraketide intermediate to produce a chalcone. B, several type III PKSs iteratively condense a long chain fatty acyl-CoA ester with a number of acetate units derived from malonyl-CoA and cyclize the linear polyketide intermediate to produce polyketides with distinct ring structures. Both ORS and ARAS consume four molecules of malonyl-CoA. ORS catalyzes decarboxylative aldol cyclization, whereas ORAS catalyzes aldol cyclization. In contrast, ArsC condenses a long chain acyl-CoA ester with two or three molecules of malonyl-CoA to produce triketide or tetraketide 2-pyrone. ORS also produces triketide and tetraketide pyrones depending on the CoA ester substrate used. ARS and ARSA produce tetraketide alkylresorcinols and alkylresorcylic acids, respectively.