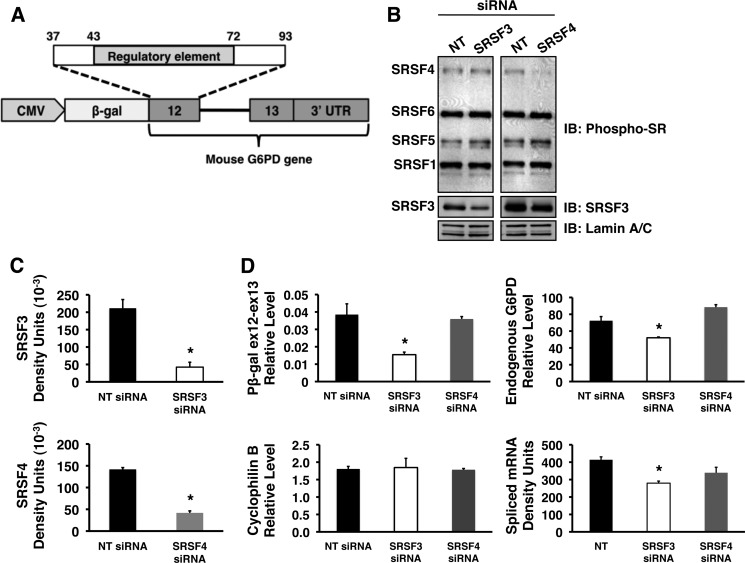
FIGURE 5.
siRNA-mediated depletion of SRSF3 reduces the splicing of a G6PD reporter and the endogenous G6PD mRNA. HepG2 cells stably expressing a splicing reporter were transiently transfected with siRNA pools directed against SRSF3, SRSF4, or with a nontargeting control siRNA (NT) for 48 h, after which the cells were harvested for isolation of total RNA and preparation of whole cell protein lysate. A, schematic representation of the splicing reporter, pβ-gal ex12-ex13. The construct contains mouse genomic DNA encompassing exon 12 (nucleotides 37–93), intron 12, and exon 13 of the G6PD gene ligated to β-galactosidase and under the transcriptional control of a CMV promoter. B, representative Western blot of whole cell lysate probed with antibodies against phosphorylated SR proteins (top panels), SRSF3 (middle panels), and lamin A/C (bottom panels). C, quantitation of SRSF3 and SRSF4 knockdown for three separate siRNA experiments in HepG2 cells. The asterisk indicates a significant difference (p < 0.05). D, total RNA isolated from HepG2 cells following knockdown was used to measure the amounts of RNA for the splicing reporter (pβ-gal ex12-ex13), the endogenous G6PD transcript, cyclophilin B, and the amount of spliced reporter RNA (spliced RNA). The asterisk indicates a significant difference (p < 0.05), n = 3 independent knockdown experiments. IB, immunoblot.