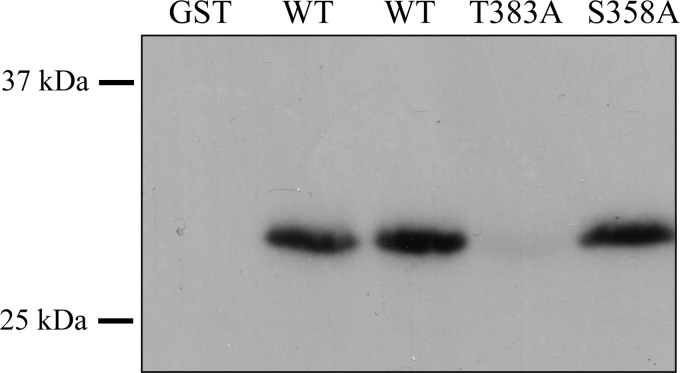
FIGURE 3.
Threonine 383 is necessary for PKCϵ phosphorylation of hTASK-1. The distal 45 residues of hTASK-1 were fused to GST, purified, and exposed to recombinant PKCϵ in the presence of [γ-32P]ATP. Additional constructs were created that contained mutations of the putative phosphorylation sites in the channel. These constructs were also incubated with the kinase. The reaction products were resolved by SDS-PAGE, blotted to nitrocellulose, and analyzed by exposure to x-ray film. The presence of radioactivity in the bands indicates the incorporation of 32P, and the result indicates that Thr-383 was required for phosphorylation by PKCϵ.