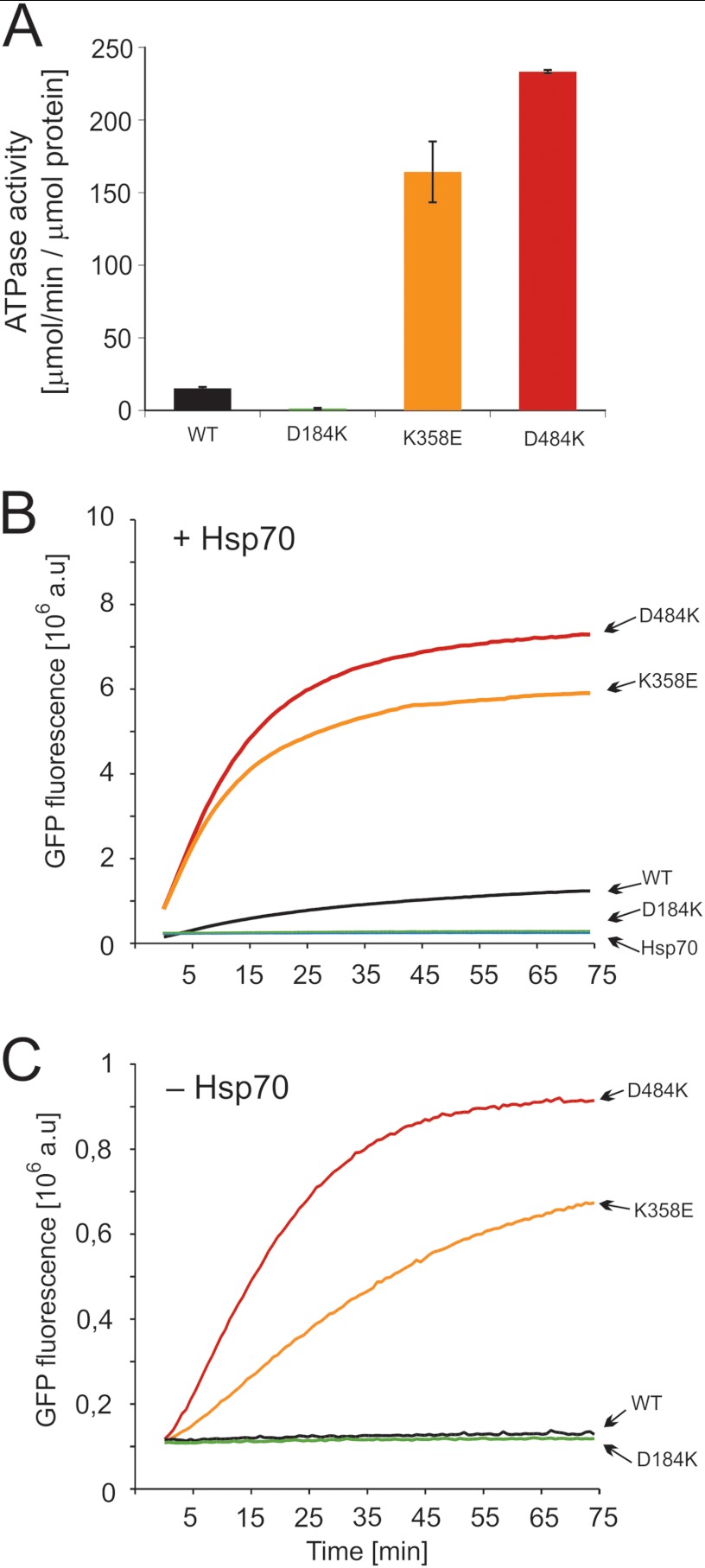
FIGURE 4.
Biochemical properties of the Hsp104 variants. A, shown is ATPase activity of Hsp104 and its variants. Data are the means (±S.D.) of three independent experiments. B, renaturation of thermally denatured GFP by purified variants of Hsp104 and wild type protein at 2 μm concentration is shown. Reactivation was performed in the presence of the Hsp70 chaperone system (Ssa1 (3 μm) and Ydj1 (1 μm)). C, shown is Hsp70-independent reactivation of thermally denatured GFP by variants of Hsp104 at 2 μm concentration. The amount of native GFP used in experiments in panels B and C corresponds to 8 × 106 fluorescence (absorbance units (a.u.)).