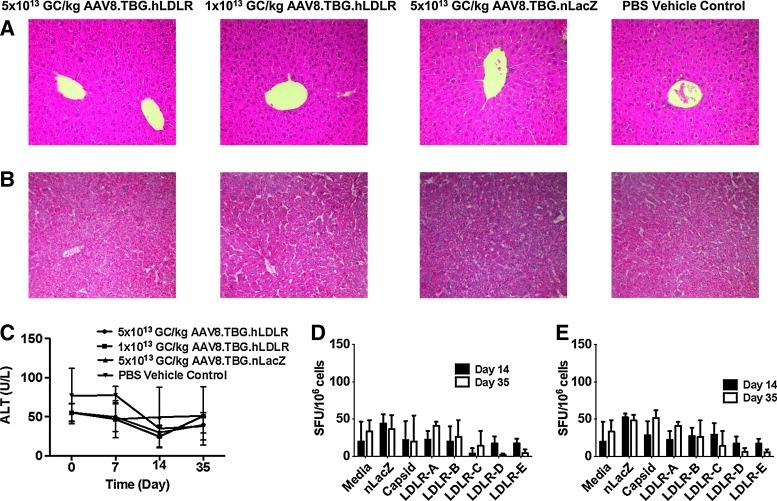
FIG. 3.
Safety of AAV8.TBG.hLDLR in LA-DKO/hApoB-Tg mice. (A) Hematoxylin–eosin (H&E) and (B) oil red O staining of samples from mice 35 days after vector administration. (C) Serum alanine aminotransferase (ALT) levels were monitored over a 35-day period. Lymphocytes were harvested from the (C) liver and (D) spleen of mice treated with 1×1012 AAV8.TBG.hLDLR or 1×1012 AAV8.TBG.nLacZ and stimulated with either the immunodominant nLacZ epitope or a human LDLR peptide library to determine the transgene-specific response. Cells were stimulated with the capsid peptide library of AAV8 to determine the capsid response. Data represent means±SD for four mice per group.