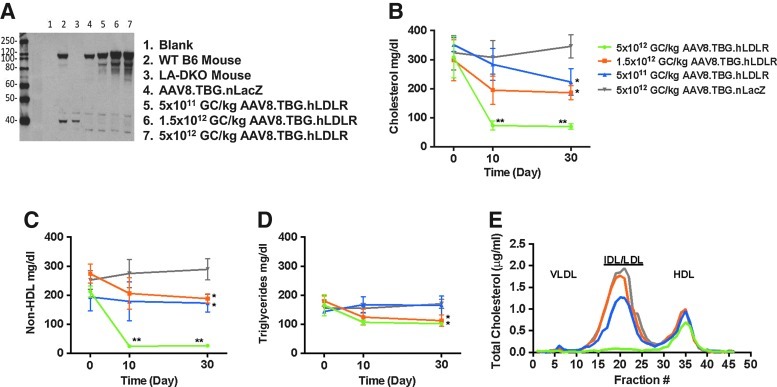
FIG. 4.
A higher dose of AAV8.TBG.hLDLR is required to achieve full correction in the humanized form of heterozygous FH mice (Ldlr+/–Apobec1–/–ApoB+/+). (A) Western blots examining hLDLR protein expression in liver lysates of representative mice 30 days after vector administration. (B) Plasma cholesterol, non-HDL cholesterol (C), and triglyceride (D) levels were examined in heterozygous mice after treatment with AAV8.hLDLR (n=6 animals per dose group). Each point represents the mean±SD. *p<0.05, **p<0.01. (E) Pooled mouse plasma from AAV-injected LAHB mice (n=6 per dose group) was analyzed by FPLC fractionation and the cholesterol content of each fraction was determined. IDL, intermediate-density lipoprotein; VLDL, very low-density lipoprotein.