Abstract
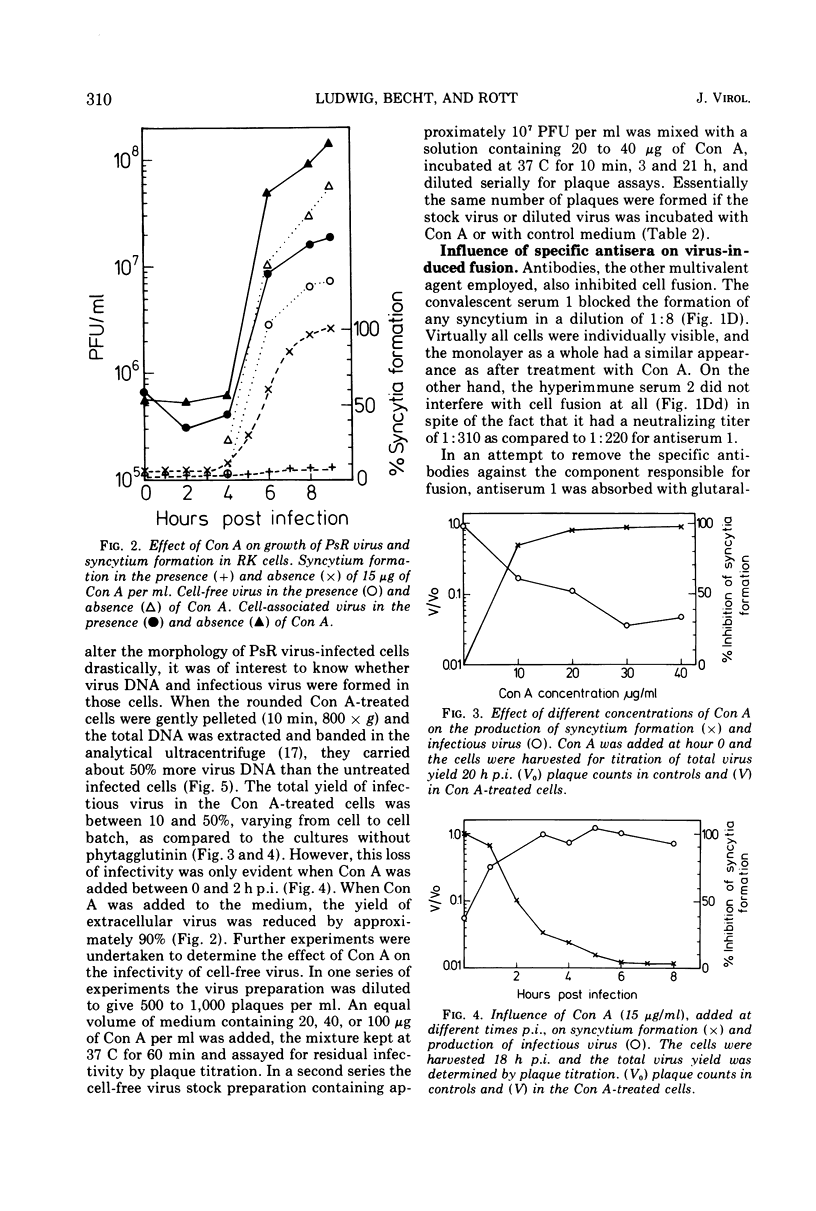
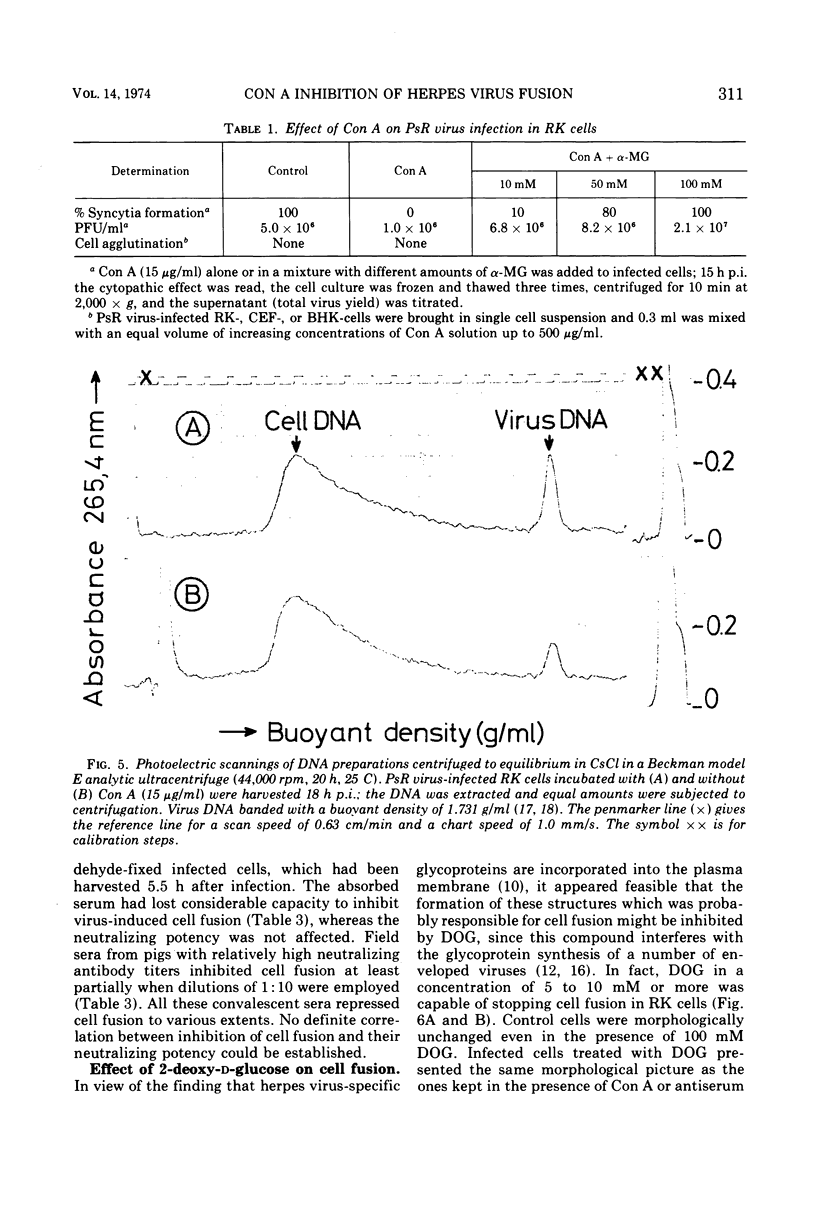
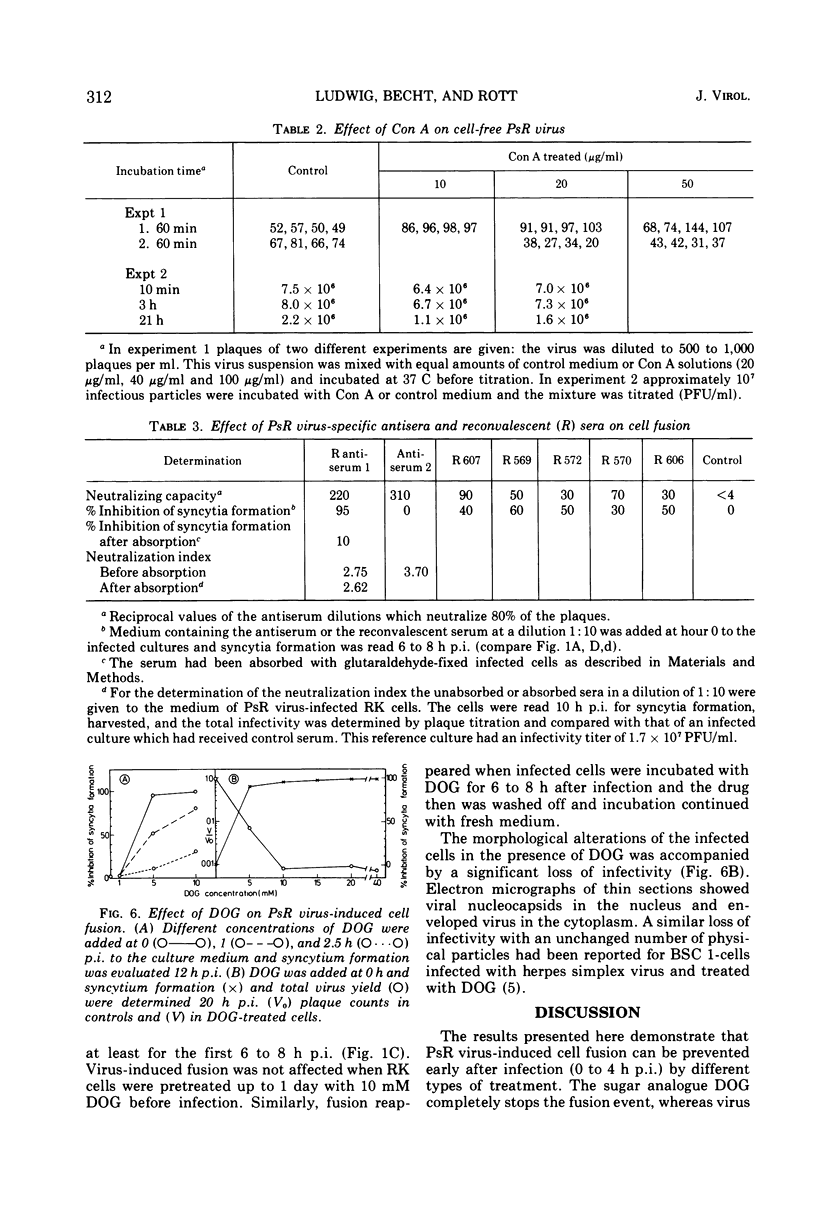
Pseudorabies virus-induced cell fusion in rabbit kidney cells can be prevented by Concanavalin A added early after infection. The infected cells are not agglutinated and the infectivity of cell-free virus is not reduced. Sera from productively infected animals also inhibit polykaryocytosis, whereas a hyperimmune serum directed against virus structural components has no effect. 2-Deoxy-d-glucose reversibly disturbs virus-induced fusion and reduces significantly the virus infectivity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becht H., Rott R., Klenk H. D. Effect of Concanavalin A on cells infected with enveloped RNA viruses. J Gen Virol. 1972 Jan;14(1):1–8. doi: 10.1099/0022-1317-14-1-1. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Studies on the biogenesis of herpesvirus envelope. Nature. 1972 Jan 21;235(5334):165–166. doi: 10.1038/235165a0. [DOI] [PubMed] [Google Scholar]
- Borgen H. C., Espensen L. Immunizing effect of photoinactivated pseudorabies virus. Zentralbl Bakteriol Orig. 1970;214(1):13–16. [PubMed] [Google Scholar]
- Chen J. H., Purchase H. G. Surface antigen on chick kidney cells infected with the herpesvirus of Marek's disease. Virology. 1970 Feb;40(2):410–412. doi: 10.1016/0042-6822(70)90421-6. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Falke D., Kahl G. F. The inhibitory effect of compound 48-80 on the formation of giant cells induced by herpesvirus hominis. J Gen Virol. 1971 Mar;10(3):273–277. doi: 10.1099/0022-1317-10-3-273. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Levitan D. B., Blough H. A. Effect of 2-deoxy-D-glucose on cell fusion induced by Newcastle disease and herpes simplex viruses. Virology. 1973 Sep;55(1):193–201. doi: 10.1016/s0042-6822(73)81021-9. [DOI] [PubMed] [Google Scholar]
- HAMADA C., KAPLAN A. S. KINETICS OF SYNTHESIS OF VARIOUS TYPES OF ANTIGENIC PROTEINS IN CELLS INFECTED WITH PSEUDORABIES VIRUS. J Bacteriol. 1965 May;89:1328–1334. doi: 10.1128/jb.89.5.1328-1334.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Barron A. L. Surface antigens produced by Herpesviruses Varicella-Zoster virus. Infect Immun. 1973 Jul;8(1):48–521. doi: 10.1128/iai.8.1.48-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBOURNE E. D. Inhibition of influenza virus multiplication with a glucose antimetabolite (2-deoxy-D-glucose). Nature. 1959 Jan 24;183(4656):271–272. doi: 10.1038/183271b0. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus, VI. Characterization of the proteins of the viral membrane. Proc Natl Acad Sci U S A. 1970 Jul;66(3):799–806. doi: 10.1073/pnas.66.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Pearson G., Nadkarni J. S., Nadkarni J. J., Klein E., Henle G., Henle W., Clifford P. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968 Nov 1;128(5):1011–1020. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Ludwig H. O., Biswal N., Benyesh-Melnick M. Studies on the relatedness of herpesviruses through DNA-DNA hybridization. Virology. 1972 Jul;49(1):95–101. doi: 10.1016/s0042-6822(72)80010-2. [DOI] [PubMed] [Google Scholar]
- Ludwig H. Untersuchungen am genetischen Material von Herpesviren. II. Genetische Verwantschaft verschiedener hepesviren. Med Microbiol Immunol. 1972;157(3):212–238. doi: 10.1007/BF02121162. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972 Nov;50(2):507–515. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Surface glycopeptides in the envelope of herpes simplex virions. Virology. 1972 Oct;50(1):277–279. doi: 10.1016/0042-6822(72)90371-6. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- Rott R., Becht H., Klenk H. D., Scholtissek C. Interactions of concanavalin A with the membrane of infleunza virus infected cells and with envelope components of the virus particle. Z Naturforsch B. 1972 Mar;27(3):227–233. doi: 10.1515/znb-1972-0303. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Lowry S., Rawls W. E., Melnick J. L., McMillan V. Detection of early cell surface changes in herpes simplex virus infected cells by agglutination with concanavalin A. J Gen Virol. 1972 Apr;15(1):93–97. doi: 10.1099/0022-1317-15-1-93. [DOI] [PubMed] [Google Scholar]




