Figure 2.
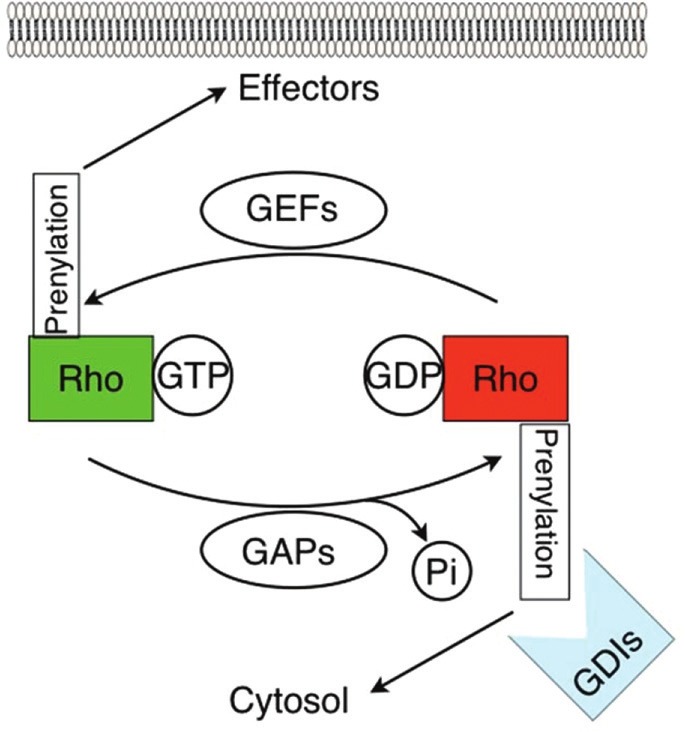
Regulation of RhoGTPase Activity. Statin-induced endothelial cell signaling and activation is mediated in part by Rho GTPases. These proteins operate as molecular switches, toggling between a GTP-bound active state (green) and a GDP-bound inactive state (red). In turn, these states may be regulated by guanine exchange factors (GEFs) and GTPase activating proteins (GAPs). Prenylation regulates GTPase function either by promoting their association with membranes where many downstream effector targets and GEFs are located, or by increasing their association with guanine dissociation inhibitors (GDIs) which favors GDP-binding and inactivation of RhoGTPases localized to the cytosol. Statin inhibition of protein prenylation leads to decreased RhoGTPase membrane association and thus decreased activation by GEFs at the membrane. However, statins also augment cytosolic GTPasebinding of Rho GTPases, with unclear functional significance, indicative of the complex effects of these drugs.
