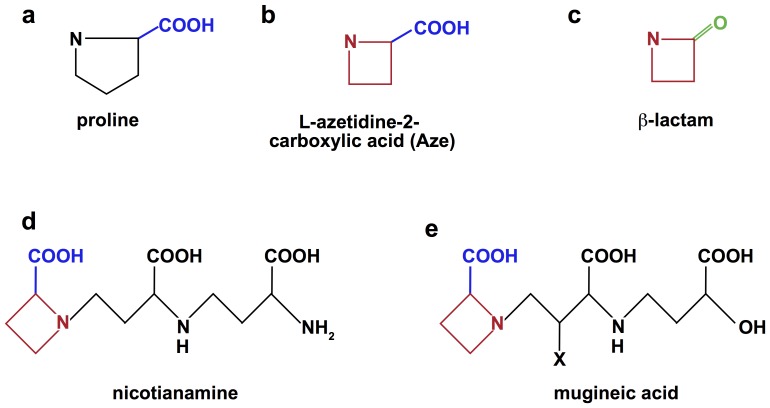
Figure 1. Structure of Proline and Related Homologues.
This figure shows the structures of proline (a), L-azetidine-2-carboxylic acid (Aze) (b), ß-lactam (c), nicotianamine (d), and mugineic acid (e). Aze is the lower homologue of proline. Its ring has only four members instead of five. Plants synthesize Aze as essential constituents of the two metal chelating molecules, nicotianamine and mugineic acid. These compounds trap metal ions in the soil and transport them to various plant parts. Aze is in particularly high concentrations in the bulbous roots of many plants, making its way into the human and lifestock food supply. Aze exerts its toxic effects by eluding the proof-reading function of the prolyl tRNA synthetases, allowing it to be misincorporated into nascent peptides or proteins in which it replaces proline. When Aze replaces proline, it can change protein structure, function, and antigenicity. This molecular mimicry is analagous to the other 4-member nitrogen containing ring of ß-lactam, which exerts its bactericidal effects by mimicking a D-Ala-D-Ala sequence of a transpeptidase, irreversibly blocking its role in bacterial cell wall synthesis. The role of Aze in human health is yet to be established.