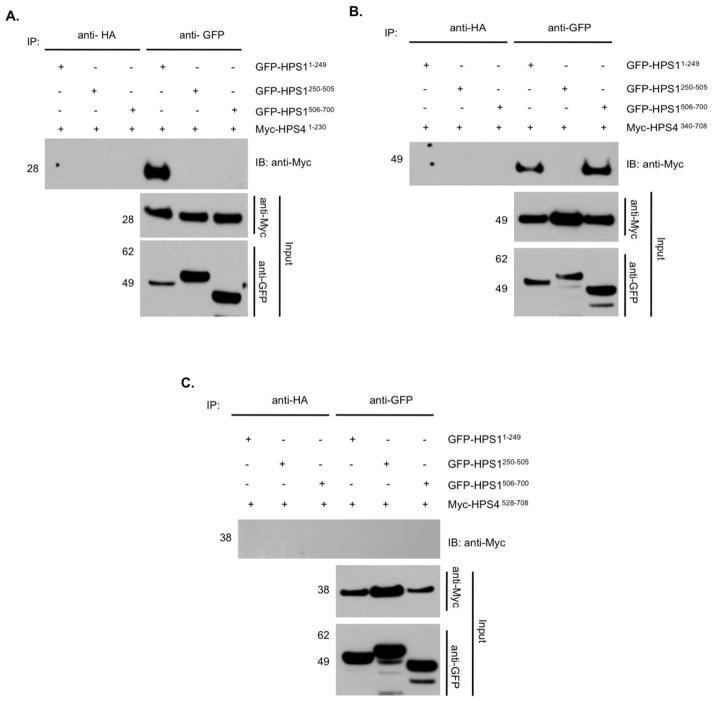
Figure 4. Mutual interaction of the N-terminal fragments of HPS1 and HPS4, while the central region of HPS4 shows divalent interaction with the N- and C- termini of HPS1.
To delineate the structural determinants of the HPS1 and HPS4 interactions, HeLa cells were co-transfected with vectors encoding Myc3- tagged HPS41–230(A), HPS4340–708 (B) or HPS4528–708 (C) truncations in combination with GFP-tagged HPS1 truncations representing N-terminal, middle and C-terminal portions of the protein. The Myc3-HPS4-N terminal region interacts with the N-terminus of HPS1, but not with the C-terminus or the middle region of this protein. Myc3-tagged HPS4340–708 interacts with both the N- and the C- termini of HPS1. The C- terminus of HPS4506–708 did not show any interaction with portions of the HPS1 protein.