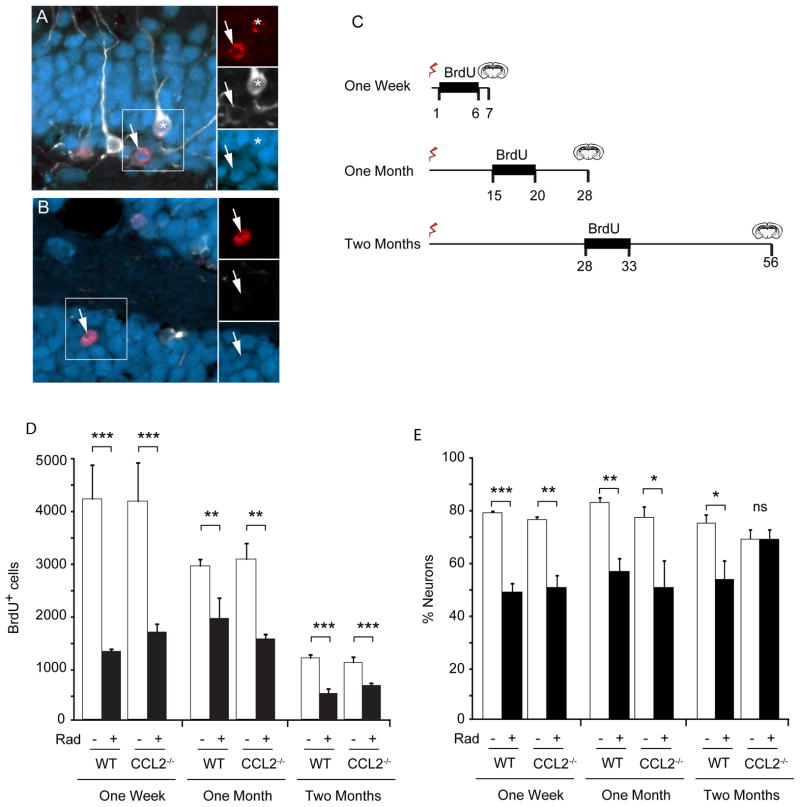
Figure 3. Impact of irradiation on neurogenesis in WT and CCL2−/− mice.
A, B Newborn cells within the dentate gyrus are labeled with BrdU (red). Initially newborn neurons express doublecortin (white) and then express NeuN (blue) as they begin to mature. The total population of newborn neurons includes immature neurons that express Dcx alone, cells that are in transition and express both Dcx and NeuN (asterisk in A), as well as more mature neurons that lose Dcx expression and become predominantly NeuN-positive (arrows in A, B). C Three BrdU labeling paradigms were used to evaluate neurogenesis at different times following irradiation. BrdU was injected daily during the indicated interval to label dividing cells and tissue then taken at the indicated times and evaluated for neurogenesis. D The total number of BrdU positive cells was determined within the GCL. Irradiation caused a significant depletion of dividing cells in both wild type (WT) and CCL2−/− animals at all time points. ANOVA revealed significant treatment effect, 1 week, F(3,12) = 12.72, p<0.001; 1 month, F(3,12) = 9.819, p<0.01; 2 month F(3,32) = 13.87, p<0.001. Posthoc Newman-Keuls test, **p < 0.01, ***p < 0.001. E The fraction of newborn neurons (cells that expressed BrdU and neuronal markers Dcx and/or NeuN) was reduced 1 week and 1 month after irradiation, but gradually recovered in CCL2−/− animals two month after irradiation. ANOVA, overall treatment effect, 1 week, F(3,12) = 39.85, p<0.001; 1 month, F(3,12) = 8.471, p<0.01; 2 month F(3,32) = 4.363, p<0.05. Posthoc Newman-Keuls, * p<0.05, **p<0.01, ***p<0.001, ns, not significant.