Figure 2.
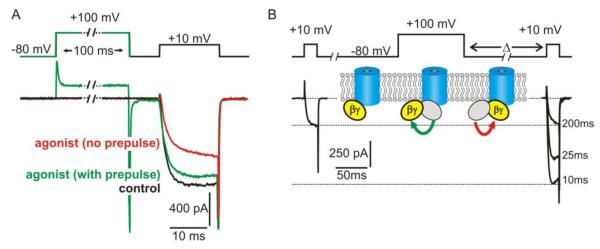
Functional effects of voltage-dependent inhibition on CaV2 channels. (A) “Whole cell” patch clamp recording of ICa from an adrenal chromaffin cell which express purinergic P2Y autoreceptors. Application of a P2Y receptor agonist (red trace) inhibited ICa compared to control conditions (black trace) with the hallmark features of voltage-dependent inhibition. Peak amplitude was reduced with prominent slowing of the activation kinetics, and both of these effects were reversed by a conditioning prepulse to +100mV (green trace). (B) Voltage-dependent relief of inhibition reflects transient dissociation of Gβγ from the channel. Shown is an example of “whole cell” ICa recorded from recombinant CaV2.2 channels expressed with β1b, α2δ and Gβγ in HEK293 cells. Gβγ produced tonic inhibition of ICa that was reversed by a conditioning prepulse to +100 mV. The magnitude of this reversal (prepulse facilitation) diminished as the interval between prepulse and test pulse (Δ) was increased (examples shown are with Δ = 10 ms, 25 ms, and 200 ms). As illustrated by the inset cartoon, prepulse facilitation is thought to reflect dissociation of Gβγ from an inhibitory binding site on the channel at the depolarized membrane potential. Upon return to the hyperpolarized membrane potential, Gβγ rebinds to (and re-inhibits) the channel. This re-inhibition of ICa is monoexponential, and the rate depends on the local concentration of Gβγ.
