Abstract
Background
Aging-related changes in important cellular pathways in the prostate may promote a permissive environment for an increased risk for prostatic disease development such as prostate cancer. Our objectives were to examine for such changes, by systematically determining the effects of growth and development and aging on proteomic profiles in different lobes of the rat prostate.
Methods
Prostate lobes (dorsolateral lobe, DL and ventral lobe, VL) were obtained from male Fisher rats of various ages representing young (4 months), mature (12 months), old (18 months), and very old (24 months). Differentially expressed proteins between age groups in each lobe were identified using a proteomic approach, isobaric Tags for Relative and Absolute Quantitation (iTRAQ). Select changes in the DL and VL were verified by immunoblot analysis.
Results
iTRAQ identified 317 proteins with high confidence. iTRAQ discovered 12 and 6 proteins significantly modulated in response to growth and development in the DL and VL, respectively, and 42 and 29 proteins significantly modulated in response to aging in the DL and VL, respectively. Proteins modulated during growth and development in the DL and VL are involved in a variety of biological processes including cell communication and development, whereas proteins modulated during aging were predominantly related to antioxidant activity and immunity. Immunoblot analysis verified age-related changes for α-1 antitrypsin, annexin A1, hypoxia up-regulated protein 1, and 78 kDa glucose-regulated protein.
Conclusions
Aging results in changes in numerous prostatic proteins and pathways which are mainly linked to inflammation and may lead to prostatic disease development.
Keywords: iTRAQ, Prostate lobes, Rat, Proteomics, Aging
Introduction
Aging is a major risk factor for prostatic diseases, including benign prostatic hypertrophy and prostate cancer (PC); PC is the most commonly diagnosed cancer among elderly American men (1,2). A number of aging-related changes are implicated in the pathogenesis of prostatic diseases, including a decline in androgen level (3), accumulation of DNA damage (4), reduction in apoptotic potential (5) and increases in inflammation (6–8) and oxidative stress (9–11). Moreover, the involvement of growth and survival factors and changes in sensitivity of the aged prostate to hormones (10,12–17) are suggested to contribute to the hyperproliferative characteristics of prostatic diseases in elderly men. In addition, changes in the molecular and cellular composition of the prostate microenvironment during aging are also suggested to be involved in the development of prostatic disorders (18–21); modulation of epithelial cells by fibroblast senescence is shown to occur via paracrine mechanisms (22,23). Despite these research findings, a comprehensive picture that links prostatic diseases to aging is still lacking.
In order to better understand the involvement of aging-related prostatic diseases, such as PC, there is a distinct need for information regarding global changes in the prostate during aging. Although several studies have performed gene expression profiles in prostate tissues from laboratory animal models (9,24,25), proteins ultimately dictate the phenotype of cells within a given tissue and may therefore, provide a better mechanistic insight into critical aging-related changes in the prostate.
To date, limited systematic studies (26) on the specific effects of aging on proteomic profiles of different lobes of the prostate have been reported. To address this need, we have examined proteomic profile changes in different lobes (dorsolateral lobe, DL and ventral lobe, VL) of the prostate of F344 rats (well-characterized as both a PC and aging model (27–36)) during four stages of life based on the lifespan characteristics of this strain of rat; young (4 months), mature (12 months), old (18 months) and very old (24 months). Analyses were conducted using a multiplex mass spectrometry-based technique, isobaric Tags for Relative and Absolute Quantitation (iTRAQ) (37).
Materials and Methods
Animal experiment
Male F344 rats, aged 4, 12, 18, and 24 months, representing young, mature, old and very old age groups, respectively, based upon survival characteristics for this long-lived strain of rat, were obtained at the same time from National Institute of Aging (NIA)/Harlan and were fed NIH-31/NIA fortified diet. All rats in the four age groups Rats were housed for two weeks in solid bottom polycarbonate cages with corn cob bedding under standardized conditions (22 ± 2° C; 50 ± 10 % relative humidity; 12 h light-dark cycle) before sacrifice and tissue isolation. At the time of sacrifice, rats were weighed. Tissues, including different lobes of the prostate, were carefully dissected, rinsed with phosphate-buffered saline, weighed, and frozen in liquid nitrogen and kept at −80°C until analysis. Statistical comparison between body weights and prostate lobes between ages was performed using Student’s two-sample, two tailed t test and comparisons with a p-value smaller than 0.05 were considered significant.
Reagents and chemicals
All iTRAQ reagents and buffers were obtained from Applied Biosystems Inc. (ABI, Foster City, CA). Reagents for immunoblot assays were purchased from Invitrogen (Carlsbad, CA) and Bio-Rad Laboratories (Hercules, CA), and primary and secondary antibodies were purchased from Abcam (Cambridge, MA), Santa Cruz Biotechnology (Santa Cruz, CA), and Cell Signaling (Danvers, MA). Chemiluminescent immunodetection reagents and autoradiography films were purchased from GE Healthcare (Piscataway, NJ) and Imaging Resources, Inc. (Seattle, WA), respectively.
iTRAQ labeling experimental design
DL and VL were processed separately for each age group; DL: 4, 12, 18, & 24 months and VL: 4, 12, 18, & 24 months. For each lobe and age group, tissues from three rats were homogenized separately in standard cell lysis buffer (Cell Signaling) using a Ten-Broeck all-glass homogenizer (Wheaton, Millville, NJ). Protein concentrations were measured by Bio-Rad Protein Assay (Bradford method) and equal amounts of protein were pooled. According to the recommendations of ABI with appropriate modifications suggested by the Penn State Hershey Cancer Institute Proteomics/Mass Spectrometry Shared Resource (Hershey, PA, http://med.psu.edu/web/core/proteinsmassspectometry/protocols/itraq), using an 8-plex iTRAQ kit, 120 μg of total protein for each group was denatured with 2% SDS (sodium dodecyl sulfate), reduced with 5 mM TCEP (tris-(2-carboxyethyl)phosphine), alkylated with 84 mM iodoacetamide (Sigma-Aldrich, St. Louis, MO), digested with trypsin (5:1, Promega, Madison, WI), and lastly labeled with one of the isobaric tag reagents, mass=113–119, or 121 Da (Table 1) (37). The combined mixtures were then separated by two-dimensional-liquid chromatography (2D-LC) and analyzed by the MS technique matrix-assisted laser desorption/ionization tandem time-of-flight (MALDI TOF/TOF) as described in the Supplementary Information File 1.
Table 1.
8-Plex iTRAQ experimental design for aging rat prostate lobe samples. Three mice were pooled together for each group and 120 μg of protein from respective prostate lobes was labeled.
| Isobaric Tag | Age of Rats | Prostate Lobe |
|---|---|---|
| 113 | 4 Months | Ventral |
| 114 | 12 Months | Ventral |
| 115 | 18 Months | Ventral |
| 116 | 24 Months | Ventral |
| 117 | 4 Months | Dorsolateral |
| 118 | 12 Months | Dorsolateral |
| 119 | 18 Months | Dorsolateral |
| 121 | 24 Months | Dorsolateral |
Protein identification and quantitation
Protein identifications and quantitation were performed with tandem MS (MS/MS) data using the Paragon algorithm as implemented in Protein Pilot™ v3.0 software (ABSciex). Identifications of proteins were only accepted with a Protein Pilot™ Unused Score of >1.3 (i.e. >95% confidence in identification). In addition, accepted protein identifications were required to have a “Local False Discovery Rate” (Local FDR) estimation of ≤5%, as calculated by the PSPEP (Proteomics System Performance Evaluation Pipeline) algorithm (38) based on the rate of accumulation of “hits” from the Decoy (reversed) database. Following identification, differential expression of proteins between different age groups for each lobe was determined by calculating the weighted average log ratios of the peptides for each protein. Quantitative data were exported into Excel (Microsoft, Bellevue, WA) for further analyses. The log ratios were compared between the profiles using Student’s two-sample, two-tailed t test and proteins with a p-value less than 0.05 and an error factor (EF) less than 2 (i.e. standard deviation less than 20%) were considered significant for quantification differences. Furthermore, protein changes ≥1.2-fold (i.e. protein ratio ≥1.2 or ≤0.8-fold demonstrating an increase or decrease, respectively) between profiles were selected; this fold-change has been used in animal model studies to avoid random variations between samples (39,40). For detailed information on searching parameters for protein identification, refer to Supplementary Information File 1 and File 2.
Biological classification of proteins
Protein ontology classification was performed by the Protein ANalysis THrough Evolutionary Relationships (PANTHER) classification system (http://www.pantherdb.org/, SRI International, Menlo Park, CA). Proteins were associated with specific molecular functions and biological processes (41).
Immunoblot analysis
Total protein (45–50 μg/sample) from DL and VL from individual animals at different age groups was electrophoretically separated on NuPAGE® 4–12% Bis-Tris gradient gels (Invitrogen). Following separation, proteins were transferred to a nitrocellulose membrane (Amersham Biosciences, GE Healthcare) and blocked with 5% nonfat dried milk (Bio-Rad) for 1 hr. The membranes were then probed with the following antibodies: anti-GRP78, anti-ORP150, anti-AAT, and anti-β-actin from Santa Cruz and anti-annexin A1 from Cell Signaling. Following incubation with primary antibodies overnight at 4°C, corresponding secondary antibodies conjugated to horseradish peroxidase were added and protein bands were detected using enhanced chemiluminescence reagents (GE Healthcare) and developed with autoradiography film (Imaging Resources, Inc).
Results
Body weights
Table 2 shows the total body weights at different ages before sacrifice. Total body weight increased significantly from 4 to 12 months of age, during the growth and developmental phase of the lifespan, plateaued during maturity between 12 and 18 months of age and decreased significantly during old age (between 18 and 24 months), consistent with previous studies (11). The weight of both prostate lobes (DL and VL) increased significantly during the growth and development period and then decreased progressively from 12 months to 18 and 24 months with the lobes in the very old age group being approximately half that of mature animals.
Table 2.
Average body and prostate lobe weights of male Fisher rats.
| Age (mo) | Lifespan stage | n | Body Weight (gm) | Prostate Lobe Weight
|
|
|---|---|---|---|---|---|
| Dorsolateral (gm) | Ventral (gm) | ||||
| 4 | Young | 9 | 358 ± 9.2 | 0.273 ± 0.02 | 0.231 ± 0.02 |
| 12 | Mature | 9 | 477 ± 12.0* | 0.366 ± 0.02‡ | 0.340 ± 0.02‡ |
| 18 | Old | 9 | 459 ± 10.5 | 0.271 ± 0.02§ | 0.237 ± 0.03§ |
| 24 | Very old | 6 | 402 ± 15.1† | 0.193 ± 0.03 | 0.162 ± 0.01¶ |
Values represent the mean ± S.E.M.
Significantly different vs. 4-month-old rats
Significantly different vs. 18-month-old rats
Significantly different vs. 4-month-old rats
Significantly different vs. 12-month-old rats
Significantly different vs. 18-month-old rats.
iTRAQ analysis of DL and VL proteins of male Fisher rats at different ages
Proteins from DLs and VLs at four age groups (4, 12, 18, and 24 months) were combined in a single 8-plex iTRAQ proteomic analysis (Table 1). Between the two prostate lobes, a total of 317 proteins were identified (>95% confidence and a Local FDR of ≤5%, cf. Materials and Methods). Figure 1 shows a representative peptide MS and MS/MS spectrum of the corresponding amino acid sequence -GVDEATIIDILTK- used in the identification and quantitation of one of the proteins identified in this study, annexin A1 (ANXA1). The relative intensities of the iTRAQ reporter ions used to quantify the relative ANXA1 protein expression for each lobe and age group is also presented in Figure 1.
Figure 1.
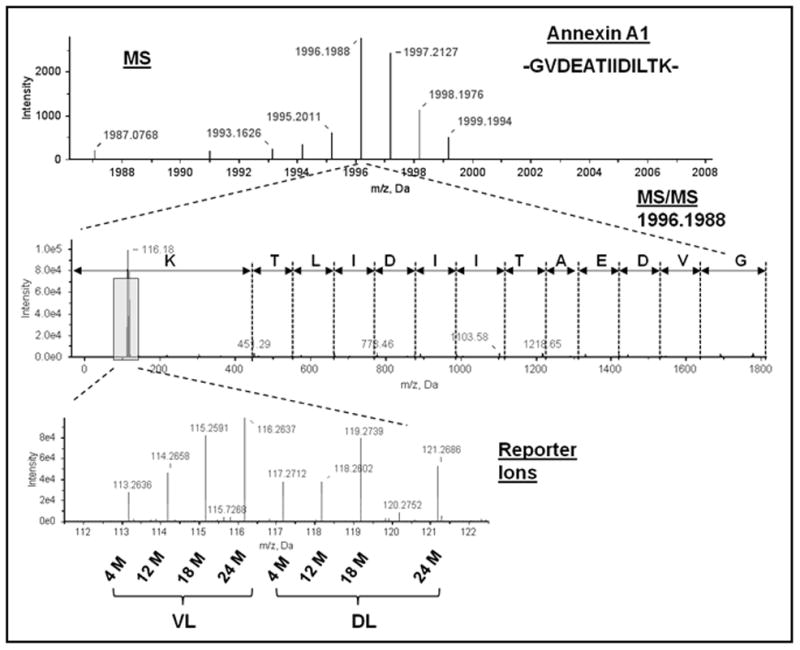
Identification of annexin A1 by MS. Representative MALDI TOF/TOF MS (upper panel) and tandem MS spectra (middle panel) used in the identification of annexin A1. The peptide sequence GVDEATIIDILTK was unique to the annexin A1 protein. Quantitation was derived from the intensities of the eight iTRAQ reporter ions (lower panel). Reporter ions 113–116 are VL collected from rats sacrificed at 4 months (4 M), 12 months (12 M), 18 months (18 M), and 24 months (24 M) and reporter ions 117–119 and 121 are DL collected from 4 M, 12 M, 18 M, and 24 M.
Among the 317 proteins identified, significant changes (p<0.05, having a fold change of ≥1.2, EF<2) during the growth and development phases of the lifespan (between 4 and 12 months of age) were observed in 12 proteins in the DL (6 up-regulated and 6 down-regulated) and 6 proteins in the VL (4 up-regulated and 2 down-regulated) (Table 3). The proteins modulated in the DL and VL are involved in a variety of biological processes including cell communication (probasin, ceruloplasmin, ANXA1), development (ezrin, prostatic glandular kallikrein-6, cysteine and glycine-rich protein 1), immunity (heat shock protein 90kDa β, 78kDa glucose-regulated protein, heat shock 70kDa protein 8, IgG γ-2A chain C region), and transport (hemopexin, albumin).
Table 3.
Proteins in the rat DL and VL that are altered significantly during growth and development (4 M to 12 M)
| * Accession No. | Protein |
† iTRAQ Protein Ratio (Fold-Change Relative to 12 Months)
|
||
|---|---|---|---|---|
| 12 M:4 M | 18 M:12 M | 24 M:12 M | ||
| A. DL Proteins up-regulated | ||||
| gi|149048531 | Ceruloplasmin (ferroxidase) | 2.58 | 1.80 | 1.94 |
| gi|81863785 | Quiescin Q6 sulfhydryl oxidase 1 | 2.07 | 0.11 | 0.12 |
| gi|38541412 | Probasin | 1.89 | 0.93 | 0.59 |
| gi|60688311 | Hemopexin | 1.75 | 1.66 | 2.63 |
| gi|228136 | Glutamine synthetase | 1.60 | 0.56 | 0.31 |
| gi|55391508 | Albumin | 1.43 | 0.81 | 1.53 |
| B. DL Proteins down-regulated | ||||
| gi|71122474 | Pyrophosphatase | 0.80 | 0.93 | 1.06 |
| gi|44890250 | Transglutaminase 4 (prostate) | 0.77 | 0.85 | 0.40 |
| gi|68067388 | Ezrin | 0.63 | 0.97 | 0.70 |
| gi|205716800 | Heat shock protein 90 kDa β (GRP94), member 1 | 0.61 | 1.32 | 0.42 |
| gi|8393206 | Cysteine and glycine-rich protein 1 | 0.55 | 1.05 | 1.39 |
| gi|157822919 | Glucosidase, α; neutral AB | 0.44 | 2.07 | 1.41 |
| C. VL Proteins up-regulated | ||||
| gi|55391508 | Albumin | 2.78 | 1.14 | 1.96 |
| gi|61556850 | Prostatic glandular kallikrein-6 | 1.87 | 0.61 | 0.05 |
| gi|121052 | IgG γ-2A chain C region | 1.80 | 4.53 | 6.43 |
| gi|6978501 | Annexin A1 | 1.54 | 2.07 | 3.13 |
| D. VL Proteins down-regulated | ||||
| gi|38303969 | 78 kDa glucose-regulated protein (GRP 78; Heat shock 70 kDa protein 5; BiP) | 0.74 | 0.50 | 0.13 |
| gi|71051777 | Heat shock 70 kDa protein 8 | 0.44 | 1.64 | 1.05 |
National Center for Biotechnology Information (NCBI).
Protein ratios in bold were statistically significant changes (p<0.05; EF<2).
Significant (p<0.05, having a fold change of ≥1.2, EF<2) aging-related changes occurring between 12 and 18 months, 12 and 24 months, or both in the DL were observed for 42 proteins (15 up-regulated and 27 down-regulated, p<0.05) (Table 4) and in the VL, 29 proteins (13 up-regulated and 16 down-regulated, p<0.05) were modulated (Table 4). Between both the DL and VL, 14 proteins were commonly identified and modulated with age. In general, the common proteins identified in the DL and VL followed similar trends, i.e. down-regulation or up-regulation with age; however, transglutaminase 4 was found down-regulated in DL and up-regulated in VL. The proteins modulated in the DL and VL during aging are involved in a variety of biological processes including generation of precursor metabolites and energy (ATP synthase subunit β, aconitate hydratase, malate dehydrogenase), motor activity (tropomyosin 2 β and α), cellular calcium-ion homeostasis (nucleobindin 2), antioxidant activity (myeloperoxidase, peroxiredoxin-6), immunity (S100 calcium-binding protein, Fc fragment of IgG binding like-protein 1, submandibular glandular kallikrein-9, α-1 inhibitor III, glutathione S-transferase μ 1 and α 3, hypoxia up-regulated protein 1, peptidylprolyl isomerase B), protein and carbohydrate metabolism (α-1 antitrypsin, transglutaminase 4, protein disulfide isomerase A3, A4 and A6, calreticulin, glutamine synthetase, creatine kinase B-type, glucosidase α, aldehyde dehydrogenase, carbonyl reductase 3), mRNA processing (heterogeneous nuclear ribonucleoproteins A2/B1), and translation (eukaryotic translation elongation factor 2).
Table 4.
Proteins in the rat DL and VL that are altered significantly during aging (12 M to 18 M or 12 M to 24 M)
| * Accession No. | Protein |
† iTRAQ Protein Ratio (Fold-Change Relative to 12 Months)
|
||
|---|---|---|---|---|
| 12 M:4 M | 18 M:12 M | 24 M:12 M | ||
| A. DL Proteins up-regulated | ||||
| gi|6978501 | Annexin A1 | 1.01 | 4.57 | 3.77 |
| gi|224493240 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 0.35 | 3.31 | 3.37 |
| gi|224472721 | Filamin α | 0.87 | 2.51 | 2.42 |
| gi|149048531 | Ceruloplasmin (Ferroxidase) | 2.58 | 1.80 | 1.94 |
| gi|62296810 | Protein disulfide-isomerase A6 | 0.55 | 1.87 | 0.34 |
| gi|55824759 | Apolipoprotein E | 4.49 | 0.95 | 5.01 |
| gi|6981010 | Hemoglobin subunit α-1/2 | 0.90 | 0.89 | 4.06 |
| gi|205830826 | Vinculin | 0.56 | 0.88 | 2.73 |
| gi|60688311 | Hemopexin | 1.75 | 1.66 | 2.63 |
| gi|51036655 | α-1 Antitrypsin | 1.09 | 0.75 | 1.98 |
| gi|157820285 | Myeloperoxidase | 0.97 | 1.72 | 1.69 |
| gi|55391508 | Albumin | 1.43 | 0.81 | 1.53 |
| gi|32363196 | Moesin | 0.87 | 1.20 | 1.46 |
| gi|8393206 | Cysteine and glycine-rich protein 1 | 0.55 | 1.05 | 1.39 |
| gi|83816939 | α-1 Inhibitor III | 1.04 | 1.03 | 1.38 |
| B. DL Proteins down-regulated | ||||
| gi|44890250 | Transglutaminase 4 (prostate) | 0.77 | 0.85 | 0.40 |
| gi|257467627 | Fc fragment of IgG binding like-protein 1 | 1.17 | 0.79 | 0.39 |
| gi|38382858 | Protein disulfide-isomerase A3 | 0.92 | 0.67 | 0.14 |
| gi|183986573 | Carbonyl reductase 3 | 0.73 | 0.60 | 0.26 |
| gi|9506445 | Carbonic anhydrase II | 1.20 | 0.59 | 0.46 |
| gi|228136 | Glutamine synthetase | 1.60 | 0.56 | 0.31 |
| gi|109504062 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 0.84 | 0.56 | 0.80 |
| gi|5902791 | Peroxiredoxin-6 | 0.78 | 0.54 | 0.27 |
| gi|56388799 | Creatine kinase B-type | 1.09 | 0.51 | 0.59 |
| gi|60391194 | Aconitate hydratase, mitochondrial | 1.35 | 0.47 | 0.74 |
| gi|8393502 | Glutathione S-transferase μ 1 | 1.42 | 0.43 | 0.99 |
| gi|71122073 | Prostatic steroid-binding protein C1 | 0.18 | 0.41 | 0.36 |
| gi|576438 | Glutathione S-transferase α 3 | 1.32 | 0.29 | 0.58 |
| gi|55605 | Aldehyde dehydrogenase, mitochondrial | 0.74 | 0.95 | 0.72 |
| gi|8393296 | Eukaryotic translation elongation factor 2 | 1.58 | 1.19 | 0.66 |
| gi|461475 | ADP/ATP translocase 1 | 0.59 | 1.07 | 0.64 |
| gi|93279423 | ATP synthase subunit β, mitochondrial | 0.95 | 0.35 | 0.60 |
| gi|78099786 | Protein disulfide-isomerase A4 | 0.88 | 0.90 | 0.54 |
| gi|81861572 | Malate dehydrogenase, cytoplasmic | 0.87 | 0.50 | 0.52 |
| gi|71051777 | Heat shock 70 kDa protein 8 | 0.97 | 0.68 | 0.50 |
| gi|55926145 | Nucleoside diphosphate kinase B | 1.37 | 0.50 | 0.48 |
| gi|205716800 | Heat shock protein 90 kDa β (GRP94), member 1 | 0.61 | 1.32 | 0.42 |
| gi|9506903 | Microseminoprotein, β (Prostate secreted seminal plasma protein) | 1.38 | 0.71 | 0.36 |
| gi|62296810 | Protein disulfide-isomerase A6 | 0.55 | 1.87 | 0.34 |
| gi|77404380 | Hypoxia up-regulated protein 1 (150 kDa oxygen-regulated protein [Orp150]) | 0.75 | 0.99 | 0.30 |
| gi|6981324 | Prolyl 4-hydroxylase, β polypeptide | 0.91 | 0.82 | 0.29 |
| gi|38541053 | Peptidylprolyl isomerase B | 1.20 | 0.79 | 0.24 |
| C. VL Proteins up-regulated | ||||
| gi|121052 | IgG γ-2A chain C region | 1.80 | 4.53 | 6.43 |
| gi|44890250 | Transglutaminase 4 (prostate) | 0.60 | 4.45 | 4.13 |
| gi|8393206 | Cysteine and glycine-rich protein 1 | 0.73 | 2.11 | 2.54 |
| gi|6978501 | Annexin A1 | 1.54 | 2.07 | 3.13 |
| gi|66730475 | Tropomyosin 2, β | 0.82 | 1.85 | 2.05 |
| gi|60688311 | Hemopexin | 3.37 | 1.58 | 1.96 |
| gi|32363196 | Moesin | 0.90 | 1.32 | 1.18 |
| gi|55824759 | Apolipoprotein E | 2.81 | 1.82 | 3.50 |
| gi|205830826 | Vinculin | 1.38 | 1.37 | 3.10 |
| gi|207350 | Tropomyosin 2, α | 1.67 | 2.19 | 2.86 |
| gi|67678416 | Keratin-8 | 0.79 | 1.57 | 1.82 |
| gi|83816939 | α-1-Inhibitor III | 1.02 | 1.08 | 1.67 |
| gi|8393502 | Glutathione S-transferase μ 1 | 1.10 | 0.91 | 1.63 |
| D. VL Proteins down-regulated | ||||
| gi|38382858 | Protein disulfide-isomerase A3 | 0.77 | 0.70 | 0.13 |
| gi|6981324 | Prolyl 4-hydroxylase, β polypeptide | 0.88 | 0.63 | 0.19 |
| gi|61556850 | Prostatic glandular kallikrein-6 | 1.87 | 0.61 | 0.05 |
| gi|38511535 | Submandibular glandular kallikrein-9 | 1.42 | 0.59 | 0.25 |
| gi|149051978 | Prostatic spermine-binding protein | 1.67 | 0.58 | 0.44 |
| gi|77404380 | Hypoxia up-regulated protein 1 (150 kDa oxygen-regulated protein [Orp150]) | 0.89 | 0.56 | 0.21 |
| gi|62296810 | Protein disulfide-isomerase A6 | 0.80 | 0.54 | 0.12 |
| gi|38303969 | 78 kDa glucose-regulated protein (GRP 78; Heat shock 70 kDa protein 5; BiP) | 0.74 | 0.50 | 0.13 |
| gi|55855 | Calreticulin | 2.78 | 0.47 | 0.10 |
| gi|219275589 | Asparaginyl-tRNA synthetase isoform 1 | 1.08 | 0.39 | 0.25 |
| gi|157822919 | Glucosidase, α; neutral AB | 1.29 | 0.38 | 0.76 |
| gi|28570182 | S100 calcium-binding protein, ventral prostate | 0.86 | 0.90 | 0.50 |
| gi|8515422 | Nucleobindin 2 | 1.04 | 0.82 | 0.49 |
| gi|8393296 | Eukaryotic translation elongation factor 2 | 0.91 | 0.84 | 0.38 |
| gi|197384345 | Coatomer protein complex subunit α | 1.32 | 0.70 | 0.30 |
| gi|72679563 | Prostatic 22 kDa glycoprotein P22K15 | 1.06 | 0.41 | 0.09 |
National Center for Biotechnology Information (NCBI).
Protein ratios in bold were statistically significant changes (p<0.05; EF<2).
The number of proteins commonly affected by either growth and development or aging change in the both the DL and VL are summarized in a venn diagram (Figure 2). While there were no proteins affected by both growth and aging in all lobes, a total of three proteins were commonly affected by growth in the DL and aging in the DL and VL (cysteine and glycine-rich protein 1, transglutaminase 4, and hemopexin), one protein was commonly affected by growth in the VL and aging in the DL and VL (annexin A1) and one protein was commonly affected by aging in the DL and growth in the VL and DL (albumin). Aging in both the DL and VL commonly impacted ten additional proteins.
Figure 2.
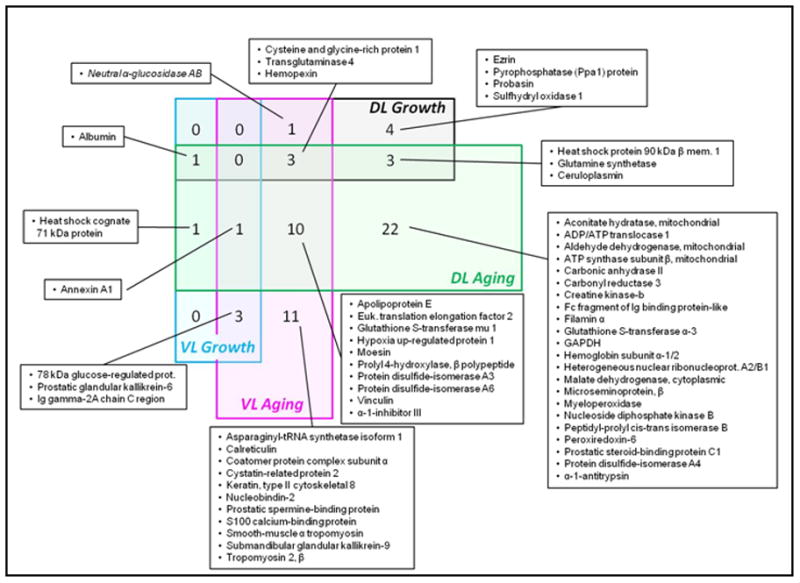
Venn diagram of proteins commonly affected during growth and development or during aging in the prostate DL and VL. Four larger rectangles represent the number of proteins being altered significantly in four major categories, DL growth and development (part of Table 3), DL aging (part of Table 4), VL growth and development (part of Table 3) and VL aging (part of Table 4). Addition of number of proteins from eight small boxes within each major category would match the total number of proteins being changed significantly in the respective category. Each small box represents the overlapping number of proteins between two, three or four major categories.
Many of the proteins commonly affected by aging in the VL and DL are involved in important cellular activities related to inflammatory processes in the prostate. The expressions of three of these proteins are commonly increased in both the VL and DL during aging and are α-1 inhibitor 3, cysteine and glycine-rich protein 1, and ANXA1. While the expression of these proteins is either decreased or unchanged during growth and development, during aging, all are enhanced in old and very old rats. The expression of three other proteins that are commonly decreased in both the VL and DL during aging are hypoxia up-regulated protein 1, prolyl-4-hydroxyl-β peptide, and protein disulfide isomerase family A, member 3. All show a similar pattern of either remaining constant or decreasing during the periods of growth and development, but decreasing during aging.
Verification of proteins found in iTRAQ by immunoblotting
Selected proteins that were changed significantly (p<0.05) during growth and development and aging in either the DL or VL or both after iTRAQ analysis were chosen for verification by immunoblot. The proteins selected included 78 kDa glucose-regulated protein (GRP78), hypoxia upregulated protein 1 (HYOU1, alternatively known as 150 kDa oxygen-regulated protein, ORP150), α-1-antitrypsin (AAT), and ANXA1. The relative expression of these proteins follows the trend observed in our iTRAQ analysis. Expressions of AAT and ANXA1 were increased with age in both the DL and VL, whereas GRP78 and ORP150 were reduced with age in the VL (Figure 3).
Figure 3.
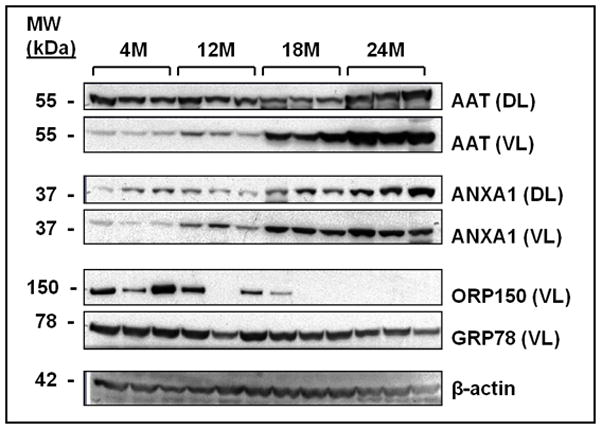
Immunoblot analysis confirming iTRAQ results for select proteins found differentially expressed in the DL and VL of aging rats. Equal amounts of protein for three rats for each age group (4 months, 4M; 12 months, 12M; 18 months, 18M; and 24 months, 24M) were loaded into each lane. The DL was probed for AAT and ANXA1 and the VL was probed for ORP150, GRP78, AAT, and ANXA1. β-actin was probed to demonstrate consisting loading.
Discussion
To our knowledge this is the first report of global protein changes in the prostate throughout the lifespan using a well-characterized and established aging model. In order to distinguish between changes which occur during growth and development phases compared to those resulting from the biological aging process, animals were sacrificed systematically throughout the lifespan at ages representing young, mature, old and very old. Since aging is often accompanied by changes in protein metabolism in different tissues, global profiling of protein expression at different ages will likely lead to valuable information on the molecular and cellular events which accompany aging in the prostate.
A key finding of this study was that the number of proteins affected during growth and development in both the DL (12 proteins) and VL (6 proteins) was substantially less than observed during aging in the DL (42 proteins) and VL (29 proteins). In general, it might be expected that global protein changes during growth and development would be substantial given the large morphologic and biochemical changes ongoing during this phase of the lifespan, whereas, aging is generally thought of as a more quiescent phase of the lifespan where more subtle and progressive changes are observed. However, in the DL and VL prostate, aging was clearly associated with more drastic changes in the proteome compared to growth and development. This could be reflective of the many pathologic biochemical changes known to occur in the aging prostate including alterations in cell proliferation and differentiation and inflammation (42). Of the proteins affected by growth, a relatively high percentage is also impacted during the aging period for both the DL (58%) and VL (67%); these proteins include cysteine and glycine-rich protein 1, heat shock protein 90 kDa β member 1, transglutaminase 4, albumin, glutamine synthetase, hemopexin and ceruloplasmin in the DL and 78 kDa glucose-regulated protein, annexin A1, prostatic glandular kallikrein-6, Ig gamma-2A chain C region in the VL.
Prostate lobe-specific alterations in protein expression
Biological processes affected in each lobe with age may account for lobe-specific disease susceptibility. For example, we identified modulated proteins in the DL related to metabolic processes, immunity, generation of energy, and biomolecule transport and proteins in the VL involved in cell communication, cell motion, developmental processes, and response to stress. In the DL, we identified microseminoprotein β (MSMB) to be down-regulated and filamin α (FLNA) to be up-regulated. The MSMB protein is a member of the immunoglobulin binding factor family synthesized by epithelial cells of the prostate and secreted into seminal plasma containing inhibin-like activity. Decreased expression of MSMB protein has been observed in prostate cancer and the gene loci associated with its expression has been linked to prostate cancer susceptibility (43,44). Our results indicated that age also affected the expression of MSMB. FLNA is involved in remodeling the cytoskeleton to effect changes in cell shape and migration. Down-regulation of FLNA mRNA has been observed during prostatic intraepithelial neoplasia development in humans (45). The fact that we observed FLNA to be up-regulated with age in the DL suggests that the expression of this protein may be important to protect against the risk of prostate cancer development. We also observed decreased expression of the antioxidant enzyme peroxiredoxin-6 in the DL, which could suggest increased oxidative stress (46).
In the VL, we identified the heat shock protein 70 chaperone GRP78 to be down-regulated during both growth and development and aging by iTRAQ and immunoblot analysis. GRP78 is found at the endoplasmic reticulum (ER) and plays a role protein folding and translocation (47,48). By microarray, Lau et al. found GRP78 to be down-regulated in VP of 16-month old rats compared to young 3-month old rats (9). Here we show for the first time that this effect appears to be aging specific.
Protein expression modulation common to both DL and VL
Commonly modulated proteins between both DL and VL were related to post-translational modifications, protein folding, cellular assembly and organization, cell death, and lipid metabolism. The shared changes between the lobes might indicate possible common molecular events associated with age that may ultimately influence prostate cancer development.
Cysteine and glycine-rich protein 1 (CSRP1), a double zinc-finger LIM protein (49), is expressed in visceral smooth muscle cells and is generally involved in cell growth and somatic differentiation. It is highly expressed in hepatocellular carcinoma (HCC) (50) and has been suggested as an important HCC biomarker because of its inactivation by aberrant methylation (51). Gene expression analyses have suggested a link between CSRP1 and prostate cancer (52). Interestingly, we found CSRP1 to be up-regulated in both prostate lobes with age.
ORP150, also known as HYOU1, is a member of the HSP 70 family of chaperone proteins like GRP78 mentioned above. ORP150 localizes to the lumen of the ER where it can function to suppress hypoxia-induced apoptotic cell death and thus, plays a crucial role in cell survival (53). In our study, we found ORP150 to decrease with age beginning at 12 months to 24 months in both the DL and VL by iTRAQ analysis. Furthermore, we confirmed this result by immunoblot analysis in the VL. In an earlier aging study using the Noble rat strain, which has a less than 2 year life span compared to other rat strains that can live past 3 years, Lam et al found ORP150 to decrease in an age-dependent manner in the VL (26) by isotope-coded affinity tags (ICAT) and MS analysis.
ANXA1 is a member of the family of calcium-dependent phospholipid binding proteins and is involved in a variety of diverse functions including regulation of membrane trafficking, signal transduction, exocytosis, inflammation, cell growth, differentiation, and apoptosis (54). Numerous studies have indicated that ANXA1 is an important player in cancer development. The absence or reduced expression of ANXA1 has been found in B-cell lymphomas (55), squamous cell carcinomas of the esophagus (56), breast cancer (57), and prostate cancer (58–61). In prostate cancer, ANXA1 is commonly reduced in all stages of development suggesting that its loss is an important event in prostate cancer initiation and progression (58–62). Accordingly, restoration or over-expression of ANXA1 in prostate cancer causes tumor suppressive functions such as apoptosis. In human androgen-sensitive prostate carcinoma cell lines, ANXA1 restoration led to activation of p38 and c-Jun N-terminal kinase (JNK), shifting the balance of signal transduction from proliferation to apoptosis (62). However, Zhang et al. found ANXA1 to be up-regulated in DL of transgenic adenocarcinoma of the mouse prostate (TRAMP) mice compared to wild-type mice (40). By iTRAQ and Western analysis, we observed a proportional increase in ANXA1 in both the DL and VL with increasing age. As it should be noted none of these rats developed prostate cancer and increased expression of ANXA1 may have provided such protection.
Prolyl-4-hydroxyl-β peptide (P4HB) is a part of the prolyl-4-hydroxylase enzyme that is involved in a multitude of functions including the formation and breakage of disulfide bonds. We found P4HB to decrease with age in our iTRAQ analysis in both DL and VL. In a previous report using ICAT and MS analysis on rat aging P4HB was down-regulated 0.48-fold in 16-month old rats compared to 3-month old rats (26). In a microarray analysis, the mRNA level of P4HB was also reported to be down-regulated in 16-month old rats and correlated well with corresponding protein levels (9,26). Here we show that the effect of P4HB down-regulation appears to be again specific and not a function of changes occurring earlier in the life span. The impact that P4HB down-regulation has with aging requires further investigation.
Transglutaminase-4 (TGM4) is one of eight Ca2+-dependent TGase isoenzymes involved in tissue protection and partakes in roles associated with skin differentiation, blood coagulation, and wound healing. TGM4 is highly expressed in the dorsolateral prostate of mice (63). In PTEN suppressed mice, loss of TGM4 expression is found in prostatic epithelium of hyperplasias and prostatic intraepithelial neoplasia related to prostate cancer development (63). Cho et al. found that human TGM4 is alternatively spliced into four mRNA transcripts in prostate tissues: TGM4 4-L, 4M-1, 4M-2, and 4–5 and further studies, demonstrated that the spliced form 4-L was lost in prostate cancer samples (64). Previous studies (65) demonstrated loss of TGM4 in metastatic prostate cancer, suggesting that TGM4 4-L is related to cancer development and progression. In addition, other studies showed 4M-1 is overexpressed in prostate cancer cells and was necessary for adhesion to endothelial cells and invasiveness (66,67). Therefore, the spliced variants of TGM4 may have different functions in cancer. We observed an age-dependent loss of TGM4 in the DL, but an increase in TGM4 in the VL of rats; however, it is unknown as to which spliced form was detected. Furthermore, Zhang et al.(40) found increased levels of TGM4 in dorsolateral prostates of TRAMP mice treated with the chemopreventive agent methylseleninic acid (MeSeA) compared to untreated TRAMP mice. This suggests that TGM4 may have tumor suppressive properties (40).
Conclusions
In conclusion, we have identified a number of proteins in the rat prostate that are differentially expressed during growth and development as well as aging and concomitantly correspond to age-related disease development such as prostate cancer. We also identified subsets of proteins with varying functions that were specific to either the DL or VL and may account for lobe-specific disease susceptibility. However, future studies will be required to further elucidate the importance of the identified proteins regarding aging and prostate cancer development.
Supplementary Material
Synopsis.
To better understand the age related risk in prostate we have examined both growth and development related and aging related changes on global proteomic profiles in different lobes (dorsolateral lobe, DL and ventral lobe, VL) of the rat prostate at 4 months (young), 12 months (mature), 18 months (old), and 24 months (very old). More proteins (42 in DL and 29 in VL) were significantly modulated in response to aging (12 months to 18 months and 24 months) than that modulated (12 in DL and 6 in VL) in response to growth and development (4 months to 12 months). Proteins modulated during growth and development in the DL and VL are involved in a variety of biological processes including cell communication and development, whereas proteins modulated during aging were predominantly related to antioxidant activity and immunity. Aging results in changes in numerous prostatic proteins and pathways which are mainly linked to inflammation and may lead to prostatic disease development.
Acknowledgments
This study was supported in part by the NCI RO1 CA127729 grant and institutional funds.
Abbreviations
- PC
Prostate cancer
- DL
Dorso-lateral prostate lobes
- VL
Ventral prostate lobes
- iTRAQ
isobaric Tags for Relative and Absolute Quantitation
- ABI
Applied Biosystems Inc
- SDS
sodium dodecyl sulfate
- TCEP
tris-(2-carboxyethyl)phosphine
- 2D-LC
Two-dimensional liquid chromatography
- MALDI TOF/TOF MS
matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass spectrometry
- FDR
False Discovery Rate
- PSEP
Proteomics System Performance Evaluation Pipeline
- EF
error factor
- PANTHER
Protein Analysis THrough Evolutionary Relationships
- ANXA1
annexin A1
- GRP78
78 kDa glucose-regulated protein
- HYOU1
hypoxia upregulated protein 1
- ORP150
150 kDa oxygen-regulated protein
- AAT
α-1-antitrypsin
- MSMB
microseminoprotein β
- FLNA
filamin α
- CSRP1
cysteine and glycine-rich protein 1
- HCC
hepatocellular carcinoma
- ICAT
isotope-coded affinity tags
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- P4HB
prolyl-4-hydroxyl-β peptide
- TGM4
transglutaminase-4
- NCBI
National Center for Biotechnology Information
Footnotes
Supporting Information Available:
Supplementary information for experimental procedures and protein identification and quantification for iTRAQ data is provided in Supplementary Information Files 1 and 2.
References
- 1.Cancer facts and figures. American Cancer Society; 2012. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Isaacs JT. The aging ACI/Seg versus Copenhagen male rat as a model system for the study of prostatic carcinogenesis. Cancer Res. 1984;44(12 Pt 1):5785–5796. [PubMed] [Google Scholar]
- 4.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61(16):6025–6028. [PubMed] [Google Scholar]
- 5.Banerjee S, Banerjee PP, Brown TR. Castration-induced apoptotic cell death in the Brown Norway rat prostate decreases as a function of age. Endocrinology. 2000;141(2):821–832. doi: 10.1210/endo.141.2.7339. [DOI] [PubMed] [Google Scholar]
- 6.Anim JT, Udo C, John B. Characterisation of inflammatory cells in benign prostatic hyperplasia. Acta Histochem. 1998;100(4):439–449. doi: 10.1016/S0065-1281(98)80040-8. [DOI] [PubMed] [Google Scholar]
- 7.Banu NA, Azim FA, Kamal M, Rumi MA, Barua AR, Khan KH. Inflammation and glandular proliferation in hyperplastic prostates: association with prostate specific antigen value. Bangladesh Med Res Counc Bull. 2001;27(3):79–83. [PubMed] [Google Scholar]
- 8.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau KM, Tam NN, Thompson C, Cheng RY, Leung YK, Ho SM. Age-associated changes in histology and gene-expression profile in the rat ventral prostate. Lab Invest. 2003;83(5):743–757. doi: 10.1097/01.lab.0000069519.06988.24. [DOI] [PubMed] [Google Scholar]
- 10.Morrissey C, Buser A, Scolaro J, O’Sullivan J, Moquin A, Tenniswood M. Changes in hormone sensitivity in the ventral prostate of aging Sprague-Dawley rats. J Androl. 2002;23(3):341–351. [PubMed] [Google Scholar]
- 11.Suzuki K, Oberley TD, Pugh TD, Sempf JM, Weindruch R. Caloric restriction diminishes the age-associated loss of immunoreactive catalase in rat prostate. Prostate. 1997;33(4):256–263. doi: 10.1002/(sici)1097-0045(19971201)33:4<256::aid-pros6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee PP, Banerjee S, Brown TR. Increased androgen receptor expression correlates with development of age-dependent, lobe-specific spontaneous hyperplasia of the brown Norway rat prostate. Endocrinology. 2001;142(9):4066–4075. doi: 10.1210/endo.142.9.8376. [DOI] [PubMed] [Google Scholar]
- 13.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001;159(6):2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkonen PL, Makela SI, Valve EM, Karhukorpi EK, Vaananen HK. Differential regulation of carbonic anhydrase II by androgen and estrogen in dorsal and lateral prostate of the rat. Endocrinology. 1991;128(6):3219–3227. doi: 10.1210/endo-128-6-3219. [DOI] [PubMed] [Google Scholar]
- 15.Prins GS, Jung MH, Vellanoweth RL, Chatterjee B, Roy AK. Age-dependent expression of the androgen receptor gene in the prostate and its implication in glandular differentiation and hyperplasia. Dev Genet. 1996;18(2):99–106. doi: 10.1002/(SICI)1520-6408(1996)18:2<99::AID-DVG2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Ruffion A, Al-Sakkaf KA, Brown BL, Eaton CL, Hamdy FC, Dobson PR. The survival effect of prolactin on PC3 prostate cancer cells. Eur Urol. 2003;43(3):301–308. doi: 10.1016/s0302-2838(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Visconti G, Herrero L, Rabadan M, Pereira I, Ruiz-Torres A. Ageing and prostate: age-related changes in androgen receptors of epithelial cells from benign hypertrophic glands compared with cancer. Mech Ageing Dev. 1995;82(1):19–29. doi: 10.1016/0047-6374(95)01593-o. [DOI] [PubMed] [Google Scholar]
- 18.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118(Pt 3):485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66(2):794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 23.Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005;4(6):291–298. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 24.Reyes I, Reyes N, Iatropoulos M, Mittelman A, Geliebter J. Aging-associated changes in gene expression in the ACI rat prostate: Implications for carcinogenesis. Prostate. 2005;63(2):169–186. doi: 10.1002/pros.20164. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita S, Suzuki S, Nomoto T, Kondo Y, Wakazono K, Tsujino Y, Sugimura T, Shirai T, Homma Y, Ushijima T. Linkage and microarray analyses of susceptibility genes in ACI/Seg rats: a model for prostate cancers in the aged. Cancer Res. 2005;65(7):2610–2616. doi: 10.1158/0008-5472.CAN-04-2932. [DOI] [PubMed] [Google Scholar]
- 26.Lam YW, Tam NN, Evans JE, Green KM, Zhang X, Ho SM. Differential proteomics in the aging Noble rat ventral prostate. Proteomics. 2008;8(13):2750–2763. doi: 10.1002/pmic.200700986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE, Kelloff GJ, McCormick DL. Chemoprevention of rat prostate carcinogenesis by early and delayed administration of dehydroepiandrosterone. Cancer Res. 1999;59(13):3084–3089. [PubMed] [Google Scholar]
- 28.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130(6):3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 29.Wylot M, Laszczynska M, Sluczanowska-Glabowska S, Piasecka M. Aging process of epithelial cells of the rat prostate lateral lobe in experimental hyperprolactinemia induced by haloperidol. Rocz Akad Med Bialymst. 2004;49 (Suppl 1):111–113. [PubMed] [Google Scholar]
- 30.Wilson MJ, Sinha AA, Powell JE, Estensen RD. Plasminogen activator activities in the developing rat prostate. Biol Reprod. 1988;38(3):723–731. doi: 10.1095/biolreprod38.3.723. [DOI] [PubMed] [Google Scholar]
- 31.Kwong J, Chan FL, Jiang S, Guo Y, Imasato Y, Sakai H, Koropatnick J, Chin JL, Xuan JW. Differential expression of PSP94 in rat prostate lobes as demonstrated by an antibody against recombinant GST-PSP94. J Cell Biochem. 1999;74(3):406–417. [PubMed] [Google Scholar]
- 32.Banerjee S, Banerjee PP, Zirkin BR. Cell proliferation in the dorsal and lateral lobes of the rat prostate during postnatal development. J Androl. 1993;14(5):310–318. [PubMed] [Google Scholar]
- 33.Ghatak S, Ho SM. Age-related changes in the activities of antioxidant enzymes and lipid peroxidation status in ventral and dorsolateral prostate lobes of noble rats. Biochem Biophys Res Commun. 1996;222(2):362–367. doi: 10.1006/bbrc.1996.0749. [DOI] [PubMed] [Google Scholar]
- 34.Hikosaka A, Asamoto M, Hokaiwado N, Kato K, Kuzutani K, Kohri K, Shirai T. Inhibitory effects of soy isoflavones on rat prostate carcinogenesis induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 2004;25(3):381–387. doi: 10.1093/carcin/bgh031. [DOI] [PubMed] [Google Scholar]
- 35.Reznik G, Hamlin MH, 2nd, Ward JM, Stinson SF. Prostatic hyperplasia and neoplasia in aging F344 rats. The Prostate. 1981;2(3):261–268. doi: 10.1002/pros.2990020304. [DOI] [PubMed] [Google Scholar]
- 36.Lucia MS, Bostwick DG, Bosland M, Cockett AT, Knapp DW, Leav I, Pollard M, Rinker-Schaeffer C, Shirai T, Watkins BA. Workgroup I: rodent models of prostate cancer. The Prostate. 1998;36(1):49–55. doi: 10.1002/(sici)1097-0045(19980615)36:1<49::aid-pros9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Bortner JD, Jr, Richie JP, Jr, Das A, Liao J, Umstead TM, Stanley A, Stanley BA, Belani CP, El-Bayoumy K. Proteomic profiling of human plasma by iTRAQ reveals down-regulation of ITI-HC3 and VDBP by cigarette smoking. Journal of proteome research. 2011;10(3):1151–1159. doi: 10.1021/pr100925p. [DOI] [PubMed] [Google Scholar]
- 38.Tang WH, Shilov IV, Seymour SL. Nonlinear fitting method for determining local false discovery rates from decoy database searches. J Proteome Res. 2008;7(9):3661–3667. doi: 10.1021/pr070492f. [DOI] [PubMed] [Google Scholar]
- 39.Kassie F, Anderson LB, Scherber R, Yu N, Lahti D, Upadhyaya P, Hecht SS. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67(13):6502–6511. doi: 10.1158/0008-5472.CAN-06-4438. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Wang L, Anderson LB, Witthuhn B, Xu Y, Lu J. Proteomic profiling of potential molecular targets of methyl-selenium compounds in the transgenic adenocarcinoma of mouse prostate model. Cancer Prev Res (Phila) 2010;3(8):994–1006. doi: 10.1158/1940-6207.CAPR-09-0261. [DOI] [PubMed] [Google Scholar]
- 41.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13(9):2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sampson N, Untergasser G, Plas E, Berger P. The ageing male reproductive tract. The Journal of pathology. 2007;211(2):206–218. doi: 10.1002/path.2077. [DOI] [PubMed] [Google Scholar]
- 43.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF. Multiple newly identified loci associated with prostate cancer susceptibility. Nature genetics. 2008;40(3):316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 44.Reeves JR, Dulude H, Panchal C, Daigneault L, Ramnani DM. Prognostic value of prostate secretory protein of 94 amino acids and its binding protein after radical prostatectomy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(20 Pt 1):6018–6022. doi: 10.1158/1078-0432.CCR-06-0625. [DOI] [PubMed] [Google Scholar]
- 45.Ashida S, Nakagawa H, Katagiri T, Furihata M, Iiizumi M, Anazawa Y, Tsunoda T, Takata R, Kasahara K, Miki T, Fujioka T, Shuin T, Nakamura Y. Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Research. 2004;64(17):5963–5972. doi: 10.1158/0008-5472.CAN-04-0020. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx3. 1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Research. 2005;65(15):6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 47.Haas IG. Protein-mediated protein maturation in eukaryotes. FEBS Lett. 1995;369(1):72–75. doi: 10.1016/0014-5793(95)00619-k. [DOI] [PubMed] [Google Scholar]
- 48.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, Lee AS, Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38(10):1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4(4):521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 50.Midorikawa Y, Tsutsumi S, Taniguchi H, Ishii M, Kobune Y, Kodama T, Makuuchi M, Aburatani H. Identification of genes associated with dedifferentiation of hepatocellular carcinoma with expression profiling analysis. Japanese journal of cancer research: Gann. 2002;93(6):636–643. doi: 10.1111/j.1349-7006.2002.tb01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirasawa Y, Arai M, Imazeki F, Tada M, Mikata R, Fukai K, Miyazaki M, Ochiai T, Saisho H, Yokosuka O. Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma. Oncology. 2006;71(1–2):77–85. doi: 10.1159/000100475. [DOI] [PubMed] [Google Scholar]
- 52.Fujita A, Gomes LR, Sato JR, Yamaguchi R, Thomaz CE, Sogayar MC, Miyano S. Multivariate gene expression analysis reveals functional connectivity changes between normal/tumoral prostates. BMC Syst Biol. 2008;2:106. doi: 10.1186/1752-0509-2-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozawa K, Kuwabara K, Tamatani M, Takatsuji K, Tsukamoto Y, Kaneda S, Yanagi H, Stern DM, Eguchi Y, Tsujimoto Y, Ogawa S, Tohyama M. 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J Biol Chem. 1999;274(10):6397–6404. doi: 10.1074/jbc.274.10.6397. [DOI] [PubMed] [Google Scholar]
- 54.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 55.Vishwanatha JK, Salazar E, Gopalakrishnan VK. Absence of annexin I expression in B-cell non-Hodgkin’s lymphomas and cell lines. BMC Cancer. 2004;4:8. doi: 10.1186/1471-2407-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li WJ, Wang C, Goldstein AM, Li G, Emmert-Buck MR, Taylor PR. Comprehensive characterization of annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10(18 Pt 1):6013–6022. doi: 10.1158/1078-0432.CCR-04-0317. [DOI] [PubMed] [Google Scholar]
- 57.Shen D, Chang HR, Chen Z, He J, Lonsberry V, Elshimali Y, Chia D, Seligson D, Goodglick L, Nelson SF, Gornbein JA. Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun. 2005;326(1):218–227. doi: 10.1016/j.bbrc.2004.10.214. [DOI] [PubMed] [Google Scholar]
- 58.Ornstein DK, Gillespie JW, Paweletz CP, Duray PH, Herring J, Vocke CD, Topalian SL, Bostwick DG, Linehan WM, Petricoin EF, 3rd, Emmert-Buck MR. Proteomic analysis of laser capture microdissected human prostate cancer and in vitro prostate cell lines. Electrophoresis. 2000;21(11):2235–2242. doi: 10.1002/1522-2683(20000601)21:11<2235::AID-ELPS2235>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 59.Kang JS, Calvo BF, Maygarden SJ, Caskey LS, Mohler JL, Ornstein DK. Dysregulation of annexin I protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Clin Cancer Res. 2002;8(1):117–123. [PubMed] [Google Scholar]
- 60.Smitherman AB, Mohler JL, Maygarden SJ, Ornstein DK. Expression of annexin I, II and VII proteins in androgen stimulated and recurrent prostate cancer. J Urol. 2004;171(2 Pt 1):916–920. doi: 10.1097/01.ju.0000104674.70170.cd. [DOI] [PubMed] [Google Scholar]
- 61.Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA. Dysregulation of the annexin family protein family is associated with prostate cancer progression. Am J Pathol. 2003;162(1):255–261. doi: 10.1016/S0002-9440(10)63816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsiang CH, Tunoda T, Whang YE, Tyson DR, Ornstein DK. The impact of altered annexin I protein levels on apoptosis and signal transduction pathways in prostate cancer cells. Prostate. 2006;66(13):1413–1424. doi: 10.1002/pros.20457. [DOI] [PubMed] [Google Scholar]
- 63.Thielen JL, Volzing KG, Collier LS, Green LE, Largaespada DA, Marker PC. Markers of prostate region-specific epithelial identity define anatomical locations in the mouse prostate that are molecularly similar to human prostate cancers. Differentiation. 2007;75(1):49–61. doi: 10.1111/j.1432-0436.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- 64.Cho SY, Choi K, Jeon JH, Kim CW, Shin DM, Lee JB, Lee SE, Kim CS, Park JS, Jeong EM, Jang GY, Song KY, Kim IG. Differential alternative splicing of human transglutaminase 4 in benign prostate hyperplasia and prostate cancer. Exp Mol Med. 2010;42(4):310–318. doi: 10.3858/emm.2010.42.4.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.An G, Meka CS, Bright SP, Veltri RW. Human prostate-specific transglutaminase gene: promoter cloning, tissue-specific expression, and down-regulation in metastatic prostate cancer. Urology. 1999;54(6):1105–1111. doi: 10.1016/s0090-4295(99)00298-8. [DOI] [PubMed] [Google Scholar]
- 66.Davies G, Ablin RJ, Mason MD, Jiang WG. Expression of the prostate transglutaminase (TGase-4) in prostate cancer cells and its impact on the invasiveness of prostate cancer. J Exp Ther Oncol. 2007;6(3):257–264. [PubMed] [Google Scholar]
- 67.Jiang WG, Ablin RJ, Kynaston HG, Mason MD. The prostate transglutaminase (TGase-4, TGaseP) regulates the interaction of prostate cancer and vascular endothelial cells, a potential role for the ROCK pathway. Microvasc Res. 2009;77(2):150–157. doi: 10.1016/j.mvr.2008.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


