Abstract
Microbial factors may play a role in the pathogenesis of recurrent aphthous stomatitis (RAS). Because of similarities in the characteristics of peptic ulcers and oral aphthous ulcers, it seems reasonable to hypothesize that Helicobacter pylori (H. pylori) could play a role in the development of RAS. The aim of the present study was to determine the relationship between H. pylori and RAS using the results obtained in other related studies. In the present systematic review, all of the relevant papers up to December 2011 were screened. The search was done using PubMed and the Cochrane library and out of 33, 9 articles were selected via the keywords of stomatitis, aphthous and H. pylori. Nine of the studies met the inclusion criteria. Among the selected articles, 6 were inconsistent with the association of H. pylori infection and RAS and 3 agreed to this assumption. The results of the literature indicate that there is no association between H. pylori infection and recurrent aphthous stomatitis.
Keywords: Aphthous stomatitis, Helicobacter pylori, Oral cavity, Pathogenesis
INTRODUCTION
Helicobacter pylori (H. pylori) is gram-negative, microaerophilic, spiral-shaped bacteria found in the gastric mucosa.[1–3] Infection with this organism is serious, transmissible and connected with peptic and duodenal ulcers, gastric carcinoma and non-Hodgkin's lymphomas of gastric mucosa-associated lymphoid tissue (MALT).[3–7] Based on the statistics, 20-30% of adult people in the developed countries and more than 90% of them in the developing countries are probably infected by this bacterium.[5] Being infected with this pathogen is basically asymptomatic and the person will be a carrier through his life, till the time when eradication therapy is done.[2,8] The exact mechanism by which H. pylori induces tissue injury is not clear. Some immune-mediated mechanisms are suggested. About 50-70% of H. pylori strains produce two types of cytotoxins: 1) VacA, whose action is increased by acid pH, which itself results in the degeneration of the epithelial cells of gastric mucosa and 2) CagA, which is a surface protein forming the chief infectious part of H. pylori and is related to a vaster spread. H. pylori strains have the ability to stimulate cytokine production, particularly IL-8 and to induce secretion of lymphocyte chemotactic factors,[9] that possibly causes a specific T-lymphocyte response.[10] The way this bacterium is transmitted is not yet quite clear. However, they are mostly found in contaminated foods, feces, saliva and the dental plaque of healthy individuals and also in patients with upper digestive system disease.[11] Thus, the infection can be caused via oral-oral, fecal- oral or gastro-oral through gastrointestinal refluxes.[2,3,8,12–15] Specialists believe that, dental plaque might be a bed of H. pylori and possibly cause re-infection of gastric mucosa, and that it is transient in the oral cavity or is mixed with normal oral microflora.[1–3,5,16–18]
Recurrent aphthous stomatitis represents a very common mucosal disorder whose cause is not yet fully understood. They can be found in both men and women of different ages, races and geographic areas. Probably about one in five (20%) of the general population has at least, once been afflicted with RAS. The condition is classified as minor, major and herpetiform on the basis of ulcer size and number. Age and sex, family and heredity, local trauma, stress, food allergy, drugs, hormonal changes, vitamin and zinc deficiencies, as well as, tobacco can result in RAS. Local and systemic conditions, genetic, immunological and microbial factors all may play a role in the pathogenesis of the RAS.[19] Important systemic disease interrelated with RAS are Celiac disease,[20] Behςet's disease[21] and the HIV associated RAS.[22]
As RAS and peptic ulcers related with H. pylori are immunologically mediated ulcers, and with regard to the fact that there is an association of probable anemia with both diseases[23,24] and because of similarities in the histological characteristics of gastric ulcers and oral aphthous ulcers which respond to treatment with broad-spectrum antibiotics, it seems logical to assume that H. pylori could play a role in the development of recurrent aphthous ulcers. The data regarding the potential relation between RAS and H. pylori infection are limited and controversial. Accordingly, the aim of the present study was to determine the association between H. pylori and RAS. It is worthy to note that, this review provides background knowledge for oral health track of the “Study on the Epidemiology of Psychological, Alimentary Health and Nutrition” (SEPAHAN).[25] The data of this study will determine the impact of oral health on functional gastrointestinal disorders and will be published later by the same study group.
MATERIALS AND METHODS
The present systematic review study was conducted to evaluate the association between H. pylori and RAS on the basis of the related literature and the evidences.
Study question
Our study evaluated the outcome of literature in the presence of H. pylori in RAS lesions of samples based on culture, histological evaluation, Enzyme-linked immunosorbent assay (ELISA), Urea breath test (UBT) and Polymerase chain reaction (PCR).
Search strategy
For electronic searching we used “Helicobacter pylori”, “aphthous stomatitis” as keywords for title and abstracts in MeSH word search. References of the papers were used for additional related papers.
Electronic databases
Information on association between H. pylori and RAS included up to December 2011, using PubMed searches. Thirty three studies were found after searching in PubMed. PubMed query translation was (“Helicobacter pylori” [MeSH Terms] or (“helicobacter” [All Fields] and “pylori” [All Fields]) or “Helicobacter pylori” [All Fields]) and (“stomatitis” [MeSH Terms] or “stomatitis” [All Fields]).
Inclusion criteria
The inclusion criteria for studies were as follows: the study design was important for data selection and all letters to editor, brief communications, editorials and systematic reviews were ruled out and just experimental and cross-sectional studies which probed the relationship between RAS and H. pylori infection and reported the effect size of the association were included. The only variable investigated for the inclusion of the data was RAS. Also, the primary selected articles were published in English. Exclusion criteria were insufficient or unrelated data and incorrect methodology. Also, if the article had multiple publications, we included the results only once. “Fifteen studies were selected first based on abstract, of these, nine articles were chosen by full text [Figure 1].”[4,9,13,26–31]
Figure 1.
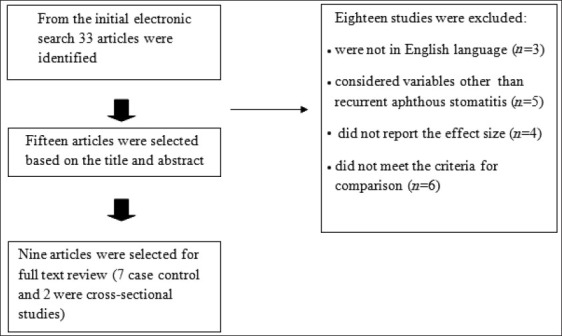
Diagram of the systematic review and searches
Data extraction
We designed a check list for data extraction including the year of publication, country, study design, sample size, diagnostic method for H. pylori detection and the results of each study. No Meta analysis was performed.
RESULTS
Nine articles were included in the study. The designs of the studies were cross-sectional and case-control. All studies were systematically appraised. The data were extracted and stratified. As shown in Table 1, the most reliable study was done by Victoria et al.,[27] with a large sample size and a very precise PCR technique (namely, nested PCR) to detect the presence of H. pylori in the oral lesions. Their technique consisted of two sets of primers, which lowers the probability of unspecific amplification and two control groups (negative and positive) associated with DNA sequencing of the PCR product. This validated the method applied. Victoria et al.,[27] arrived at the conclusion that, 38.9% of patients with RAS and 33.3% of control group were H. pylori positive, which did not give support to the assumption that H. pylori could be involved in RAS development (P>0.05).
Table 1.
Related studies of RAS and H. pylori infection that included in this systematic review
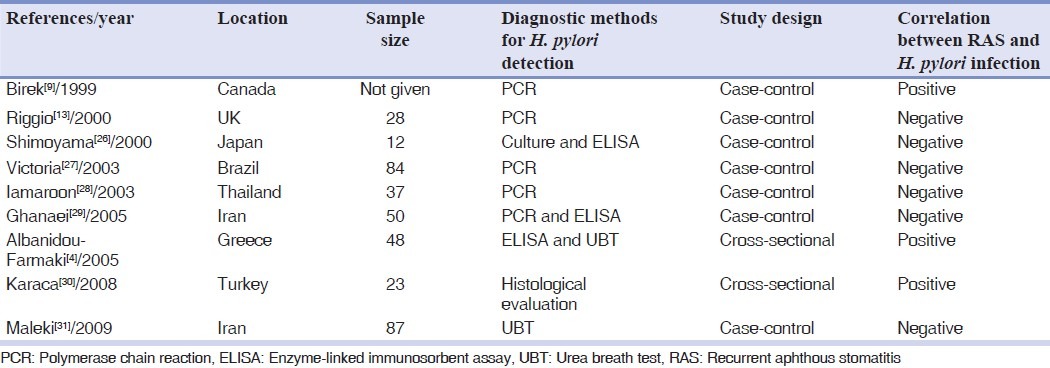
The study by Iamaroon et al.,[28] used nested PCR and samples were brushed from the oral lesions and the dorsum of the tongue of each patient. When standard PCR technique was applied, none of the samples showed positive results. However, following the application of a more sensitive method, namely, nested PCR; the researchers arrived at positive results. The negative and positive control groups were in consonance with samples from the ulcer and the tongue, yielding proper results, thus, ruling out the possibility of contamination results in the validation of the PCR. They showed that only one sample from a lesion (4.5%) was positive for H. Pylori, so they concluded that H. pylori did not play a role in the pathogenesis of RAS ulcers.[28]
Riggio et al.,[13] detected H. pylori Genomic Deoxyribonucleic acid (DNA) which had been extracted from biopsies, thus confirming successful extraction of PCR-amplifiable DNA which happened after carrying out PCR on each DNA sample with nested primers specific for the human beta-hemoglobin gene. PCR identification of H. pylori is carried out using a primer pair specific for the H. pylori 16S ribosomal RNA (rRNA) gene. Two rounds of PCR were carried out to amplify a 295-bp product, and the identity of amplified products was confirmed by DNA sequencing. H. pylori DNA was detected in 11% of RAS samples. They thus concluded that there is no certain causal role for H. pylori in RAS.[13]
Birek et al.,[9] also used PCR techniques for detecting H. pylori in swabs of RAS lesions and samples of other oral sites showed that in the RAS samples, 71.8% were found to be positive for H. pylori DNA, as detected by the PCR technique, which suggests that H. pylori may be related in most cases with RAS.[9]
Shimoyama et al.,[26] employed culture as a gold standard to investigate the relationship between H. pylori and oral mucosal ulcerations. Patients with oral mucosal ulcerative disorders were used as samples. Twelve of these samples had RAS. Serum IgG antibodies against H. pylori were probed in all cases. In their study, all of the RAS cases were culture-negative for H. pylori which suggest that H. pylori might not have a direct association with oral ulcerations.[26]
Karaca et al.,[30] did a study to research the relationship between H. pylori and RAS in addition to the effect of eradication therapy on the recurrence. All patients underwent endoscopic examination and gastric biopsy. The biopsy materials were examined histopathologically and were checked to see if they had H. pylori. The density of H. pylori was also investigated. The patients with H. pylori infection were checked up for about 1 year after the beginning of eradication therapy. The results showed that gastric mucosal H. pylori colonization was positive in 87% and negative in 13% of the patients, respectively. There were statistically significant decreases in the recurrence rate and amelioration time of RAS by eradication therapy. There were no significant correlations among the intensity of H. pylori with the recurrence rate, number, diameter, and amelioration time of the lesions in 1-year follow-up. Thus, the researchers supported this idea.[30]
Ghanaei et al.,[29] collected oral aphthous specimens by toothbrush from the patients. PCR was used to detach H. pylori in the samples of RAS lesions and other parts of the oral cavity. ELISA was also carried out in all patients to determine IgG antibody. As a result of their study, 26 patients (52%) had positive ELISA test and H. pylori DNA was obtained in only 1 patient (2%). According to the results of this study, H. pylori DNA could not be found in the aphthous ulcers of these patients.[29]
Maleki et al.,[31] had cases and control groups disregarding age and gender. UBT was used to detect H. pylori infection. It was found that, 37.2% of individuals among the RAS patients and 31.8% individuals in the control group had a positive UBT. The difference was not found to be statistically significant (P=0.597). In other words, no correlation was detected. However, the probability of a positive test was higher in the more severe cases and this should not escape attention.[31]
Farmaki et al.,[4] applied specific immune-globulin G (IgG) and immunoglobulin A (IgA) antibodies. They used the ELISA technique in the serum and the saliva of the patients. In all H. pylori carriers an eradication therapy was applied and after 2 months, the patients were checked for the existence of H. pylori (H. pylori status), after applying UBT. At entry, patient with H. pylori infection suffered from more severe symptoms compared with H. pylori negative patients (P<0.05) and after the eradication therapy, the periods between recurrence of RAS in patients who had become negative were significantly longer compared with those before treatment (P<0.001). These findings support the concept of a potential association between RAS and H. pylori.[4]
In general, the results of 6 articles showed no significant correlation between H. pylori infection and RAS and the three studies reported a significant relationship between them.
DISCUSSION
In this study, related literature on the role of H. pylori in the development of RAS was systematically reviewed. Of the 33 papers probed, 9 were related and of the selected ones, 6 were inconsistent with the association of H. pylori infection and RAS and 3 were in line with the assumption. The results mentioned in the literature showed that there is no association between H. pylori infection and RAS.
In the studies reviewed, different diagnostic methods were used to detect the existence of H. pylori in the oral ulcers. PCR was the technique mostly used in the studies (5 from 9 studies). The researchers preferred PCR because these methods have high level of accuracy. Although some believe that, as PCR is very sensitive, it might result in a series of pseudo-positive answers in some cases. The problem is that the results basically depend on the test used. For example in the PCR technique, the reported variation in the detection range of analysis may reflect variations in the prevalence of H. pylori but is more likely due to differences in the specificity and sensitivity of the primer used.[2] In other words, the PCR technique used in different studies are done with different frequencies of primers some of which are more sensitive, which causes decrease in the possibility of unspecific amplification and can contribute to a more precise detection of H. pylori. For example, in the study by Victoria et al. two sets of primers were used and the use of negative and positive controls associated with DNA sequencing, was necessary to gain more precise results[27] and in the study by Iamaroon et al.,[28] after employing the standard PCR technique, none of the samples showed positive results but following the use of a more sensitive method, nested PCR, the investigators obtained positive results. Also, by using the more sensitive technique, the possibility of amplification of other organisms or species instead of H. pylori will be decreased. However, one could consider the possibility of amplification of another species of Helicobacter such H. fennelliae, H. cinaedi, since these bacteria have also been found in human gut.[32] Also, another Helicobacter-like organism has been found in the oral cavity, such as Campylobacter rectus and C. curvus, which should not be ignored. Anyhow, in the study by Victoria et al. the DNA sequence of the PCR product obtained from the patients showed a 100% match when compared with the DNA sequence of H. pylori strain Tx30a which of course endorses the validity of their method.[33]
As culture is generally recognized as the gold standard for diagnosis of H. pylori infection Shimoyama et al. used this method to examine the association of H. pylori with oral mucosal ulceration. However, H. pylori in the oral cavity might be in a non-culturable coccoid state without productive infection, and will possibly form colonies only in particular conditions such as Herpes simplex virus (HSV) infection.[26] Some studies have suggested that H. pylori may exist in a dormant, spore-like state that can be viable but not culturable. It has been suggested that under stress and nutrient deprivation, H. pylori goes through a morphological transformation from actively spiral bacilli to inactive cocci.[34] Some authors also suggest that this morphological change could result in failure to separate H. pylori from the oral cavity.[26,35] Due to this problem, this method is not frequently used in the studies.
In a study by Ghanaei et al.[29] both ELISA and PCR tests were applied but even in samples with positive IgG, no H. pylori DNA was detected in the aphthous ulcer's samples. So, it is probable that these bacteria are not contained in the RAS. Also, Riggio et al.'s study[13] did not support the causative role of H. pylori in RAS development but the possibility that this organism may be involved in a small proportion of RAS cases could not be excluded. We can elaborate that the number of H. pylori organisms needed to cause ulcers in the stomach and oral cavity is not quite clear yet and that further research studies are needed to see if the low number of organisms in some of the samples from saliva or swabs from ulcers in some studies could be considered as factor causing RAS development.
Although, it was hypothesized that there is a relationship between H. pylori in RAS and chronic gastritis,[9] the low frequency of H. pylori in the most of studies could be partly justified by the fact that the sample patients of all studies did not have gastric disorders and were all healthy.
Also, patients who use any sort of oral rinses or systemic drugs should not be included in the study. Moreover, to avoid any form of bias, caries and periodontitis and the age and sex of all groups should be matched. In the majority of the research studies, no proper control over the sample matching was executed, which can be the cause of controversial results.
In the absence of a definitive aetiology or diagnostic test for RAS, the diagnosis of RAS in clinical practice is usually dependent on the combination of history, clinical features and histopathology.[19] The clinical assessments of RAS were not homogeneous in the related studies, whereas it goes without saying that the diagnosis of RAS should be based on a common set standard.[27] For instance, in the study by Farmaki et al. the RAS assessment was based on a scale of no symptoms, mild symptoms, moderate symptoms and severe symptoms, whereas in the research study by Victora et al. the lesions with well-circumscribed margins and surrounded by an erythematous halo, were selected. In their study, Ghanaei et al.[29] patients who had referred to the laboratory were selected as the ones diagnosed with RAS. Such variations in the methodologies could have resulted in varied results. So it is recommended that common criteria be set for RAS diagnosis.[4,27,29]
In some of the recent studies, dorsal surface of the tongue and the saliva and other parts of the oral cavity are considered to be the sources of H. pylori in the mouth[3] so it should be taken into account in the sample collection.[9]
Recurrent aphthous stomatitis is a painful situation, featured by necrotizing ulcers of the oral mucosa that persist, remit and recur for different periods. Although the disease is self limited, pain and ulceration may disable the patients, prevent them from performing their daily activity. This means that the quality of their lives will be lowered. For managing this problem good oral hygiene, appropriate toothpaste (without irritating sodium lauryl sulfate), and sedative therapy for relieving the pain[19] are recommended. However, since the exact nature of RAS remains unclear, no treatment is available at the time being. It can be hypothesized that, if H. pylori has a role in the development of RAS, we can decrease the frequency and the severity of the RAS lesions, by omitting the organism through chlorhexidine mouth rinses or irrigation and developing oral hygiene via mechanical plaque removal parallel to the triple therapy (a therapeutic period by antibiotics for digestive infections),[3] which itself results in patient's comfort and a better quality of life. The idea that, after eradication of H. pylori, the severity and recurrence frequency of RAS is decreased has been supported by Karaca et al. and Farmaki et al.[4,30]
The limitations of our systematic review were as follows: Only published papers in English were reviewed, although some related ones were published in other languages, we looked for relevant articles just in PubMed which itself can be considered a limitation. Also, we narrowed the keywords to focus on the only variable of the present study which is RAS and the related systemic conditions like Celiac disease, Behςet's disease and the Human immunodeficiency virus (HIV) associated RAS have not been considered. The reviewed literature used in the study themselves had conflicting results because of variations in the techniques applied, differences in collection of specimens, the differences in the study population and sample sizes and variations in the primers and target DNA used in the PCR assay.[3,9,18]
It can be concluded from review of the literature that, no causal relationship between H. pylori infection and recurrent aphthous stomatitis, has been established yet.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pedersen A. Are recurrent oral aphthous ulcers of viral etiology? Med Hypotheses. 1991;36:206–10. doi: 10.1016/0306-9877(91)90132-i. [DOI] [PubMed] [Google Scholar]
- 2.Kilmartin CM. Dental implications of Helicobacter pylori. J Can Dent Assoc. 2002;68:489–93. [PubMed] [Google Scholar]
- 3.Navabi N, Aramon M, Mirzazadeh A. Does the presence of the Helicobacter pylori in the dental plaque associate with its gastric infection? A meta-analysis and systematic review. Dent Res J (Isfahan) 2011;8:178–82. doi: 10.4103/1735-3327.86033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albanidou-Farmaki E, Giannoulis L, Markopoulos A, Fotiades S, Aggouridaki X, Farmakis K, et al. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005;11:22–6. doi: 10.1111/j.1601-0825.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett SA, Kowolik MJ. Oral Helicobacter pylori: Can we stomach it? Crit Rev Oral Biol Med. 2003;14:226–33. doi: 10.1177/154411130301400307. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 7.Solnick JV, Vandamme P. Taxonomy of the Helicobacter Genus. In: Mobley HL, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Washington (DC): ASM Press; 2001. Chapter 5. [PubMed] [Google Scholar]
- 8.Czesnikiewicz-Guzik M, Bielanski W, Guzik TJ, Loster B, Konturek SJ. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J Physiol Pharmacol. 2005;56(Suppl 6):77–89. [PubMed] [Google Scholar]
- 9.Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G. Detection of Helicobacter pylori in oral aphthous ulcers. J Oral Pathol Med. 1999;28:197–203. doi: 10.1111/j.1600-0714.1999.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 10.Shimoyama T, Crabtree JE. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut. 1998;43(Suppl 1):S2–5. [PMC free article] [PubMed] [Google Scholar]
- 11.De Giacomo C, Fiocca R, Villani L, Lisato L, Licardi G, Diegoli N, et al. Helicobacter pylori infection and chronic gastritis: Clinical, serological, and histologic correlations in children treated with amoxicillin and colloidal bismuth subcitrate. J Pediatr Gastroenterol Nutr. 1990;11:310–6. [PubMed] [Google Scholar]
- 12.Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health. 2002;92:1809–15. doi: 10.2105/ajph.92.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med. 2000;29:507–13. doi: 10.1034/j.1600-0714.2000.291005.x. [DOI] [PubMed] [Google Scholar]
- 14.Silva Rossi-Aguiar VP, Navarro-Rodriguez T, Mattar R, de Melo Peres MP Siqueira, Correa Barbuti R, Silva FM, et al. Oral cavity is not a reservoir for Helicobacter pylori in infected patients with functional dyspepsia. Oral Microbiol Immunol. 2009;24:255–9. doi: 10.1111/j.1399-302X.2008.00491.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown LM. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 16.Silva DG, Tinoco EM, Rocha GA, Rocha AM, Guerra JB, Saraiva IE, et al. Helicobacter pylori transiently in the mouth may participate in the transmission of infection. Mem Inst Oswaldo Cruz. 2010;105:657–60. doi: 10.1590/s0074-02762010000500009. [DOI] [PubMed] [Google Scholar]
- 17.Thomas E, Jiang C, Chi DS, Li C, Ferguson DA., Jr The role of the oral cavity in Helicobacter pylori infection. Am J Gastroenterol. 1997;92:2148–54. [PubMed] [Google Scholar]
- 18.Nguyen AM, el-Zaatari FA, Graham DY. Helicobacter pylori in the oral cavity. A critical review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:705–9. doi: 10.1016/s1079-2104(05)80304-x. [DOI] [PubMed] [Google Scholar]
- 19.Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Hayrinen-Immonen R. Recurrent aphthous ulcers today: A review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221–34. doi: 10.1006/ijom.2002.0446. [DOI] [PubMed] [Google Scholar]
- 20.Meini A, Pillan MN, Plebani A, Ugazio AG, Majorana A, Sapelli PL. High prevalence of DRW10 and DQW1 antigens in celiac disease associated with recurrent aphthous stomatitis. Am J Gastroenterol. 1993;88:972. [PubMed] [Google Scholar]
- 21.Lehner T. Oral ulceration and Behcet's syndrome. Gut. 1977;18:491–511. doi: 10.1136/gut.18.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPhail LA, Greenspan D, Greenspan JS. Recurrent aphthous ulcers in association with HIV infection. Diagnosis and treatment. Oral Surg Oral Med Oral Pathol. 1992;73:283–8. doi: 10.1016/0030-4220(92)90122-7. [DOI] [PubMed] [Google Scholar]
- 23.Haisraeli-Shalish M, Livneh A, Katz J, Doolman R, Sela BA. Recurrent aphthous stomatitis and thiamine deficiency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:634–6. doi: 10.1016/s1079-2104(96)80437-9. [DOI] [PubMed] [Google Scholar]
- 24.Sarker SA, Mahmud H, Davidsson L, Alam NH, Ahmed T, Alam N, et al. Causal relationship of Helicobacter pylori with iron-deficiency anemia or failure of iron supplementation in children. Gastroenterology. 2008;135:1534–42. doi: 10.1053/j.gastro.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Adibi P, Keshteli AH, Esmaillzadeh A, Afshar H, Roohafza H, Bagherian H, et al. The study on the epidemiology of psychological, alimentary health and nutrition (SEPAHAN): Overview of methodology. J Res Med Sci. 2012;17(5):[In Press]. [Google Scholar]
- 26.Shimoyama T, Horie N, Kato T, Kaneko T, Komiyama K. Helicobacter pylori in oral ulcerations. J Oral Sci. 2000;42:225–9. doi: 10.2334/josnusd.42.225. [DOI] [PubMed] [Google Scholar]
- 27.Victoria JM, Kalapothakis E, Silva Jde F, Gomez RS. Helicobacter pylori DNA in recurrent aphthous stomatitis. J Oral Pathol Med. 2003;32:219–23. doi: 10.1034/j.1600-0714.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 28.Iamaroon A, Chaimano S, Linpisarn S, Pongsiriwet S, Phornphutkul K. Detection of Helicobacter pylori in recurrent aphthous ulceration by nested PCR. J Oral Sci. 2003;45:107–10. doi: 10.2334/josnusd.45.107. [DOI] [PubMed] [Google Scholar]
- 29.Ghanaei MF, Asmar M, Bagherzadeh AH, Ekbataninezhad S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med Sci Monit. 2005;11:576–9. [PubMed] [Google Scholar]
- 30.Karaca S, Seyhan M, Senol M, Harputluoglu MM, Ozcan A. The effect of gastric Helicobacter pylori eradication on recurrent aphthous stomatitis. Int J Dermatol. 2008;47:615–7. doi: 10.1111/j.1365-4632.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- 31.Maleki Z, Sayyari AA, Alavi K, Sayyari L, Baharvand M. A study of the relationship between Helicobacter pylori and recurrent aphthous stomatitis using a urea breath test. J Contemp Dent Pract. 2009;10:9–16. [PubMed] [Google Scholar]
- 32.Flores BM, Fennell CL, Kuller L, Bronsdon MA, Morton WR, Stamm WE. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Campylobacter cinaedi and Campylobacter fennelliae. Infect Immun. 1990;58:3947–53. doi: 10.1128/iai.58.12.3947-3953.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: Evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–23. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Okuda K, Ishihara K, Miura T, Katakura A, Noma H, Ebihara Y. Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol Immunol. 2000;44:385–8. doi: 10.1111/j.1348-0421.2000.tb02510.x. [DOI] [PubMed] [Google Scholar]


