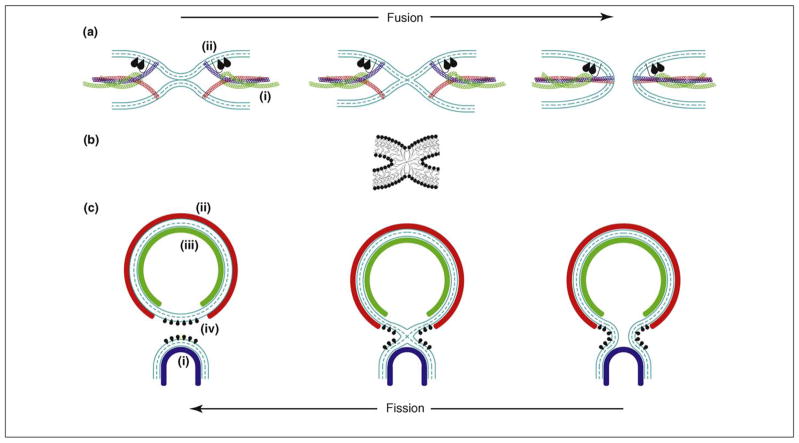
Figure 1.
Pathways and protein-driven mechanisms of membrane remodeling. (a) Protein-mediated membrane fusion. The fusion reaction (from left to right) is driven by membrane curvature, which can be generated by (i) force transmission from the SNARE complex folding into a four-helix bundle through a sufficiently rigid helical link to the transmembrane domain [53] and/or (ii) shallow hydrophobic insertions of the C2 domains of synaptotagmin or Doc2 [21,22,32,33]. The fusion pathway consists of membrane dimpling leading to the generation of curvature stresses and the establishment of point-like intermembrane contacts (left panel), fusion stalk formation (middle panel), and fusion pore formation and expansion (right panel). (b) A membrane stalk, a common intermediate structure of fusion and fission. See [73] for details regarding the computation of the stalk configuration presented. (c) Protein-mediated membrane budding and fission. The fission reaction (from right to left) is driven by the generation of a strongly curved membrane neck whose elastic energy is relaxed as a result of membrane splitting. The neck formation and stressing can be mediated by (i) membrane adhesion on a dome-like protein scaffold formed by the ESCRT-III complex (blue) [60], and/or (ii) membrane scaffolding by the outer membrane coat (e.g. COPI, COPII and viral protein ectodomain coats, red) [3] and/or (iii) scaffolding by the inner membrane coat (e.g. viral matrix, core or capsid protein coat, green), and/or (iv) by the action of shallow hydrophobic insertions.