Abstract
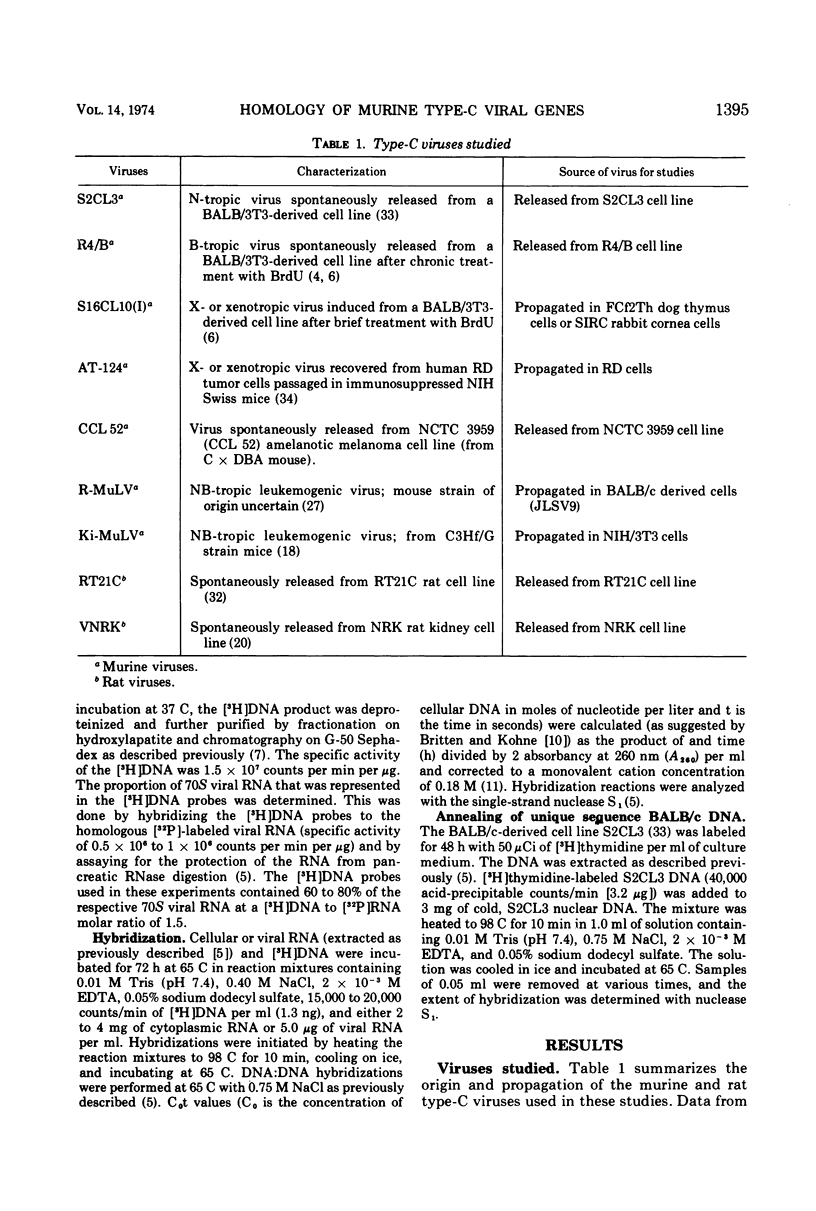
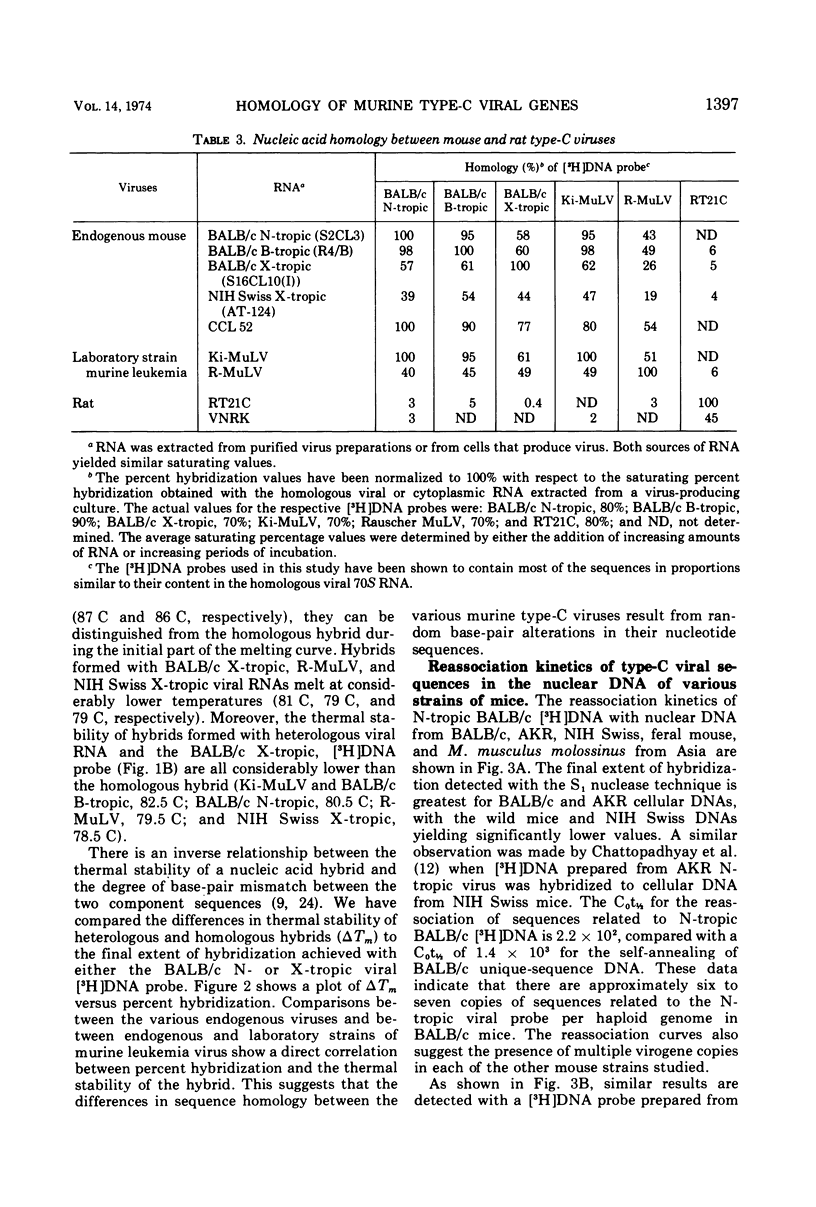
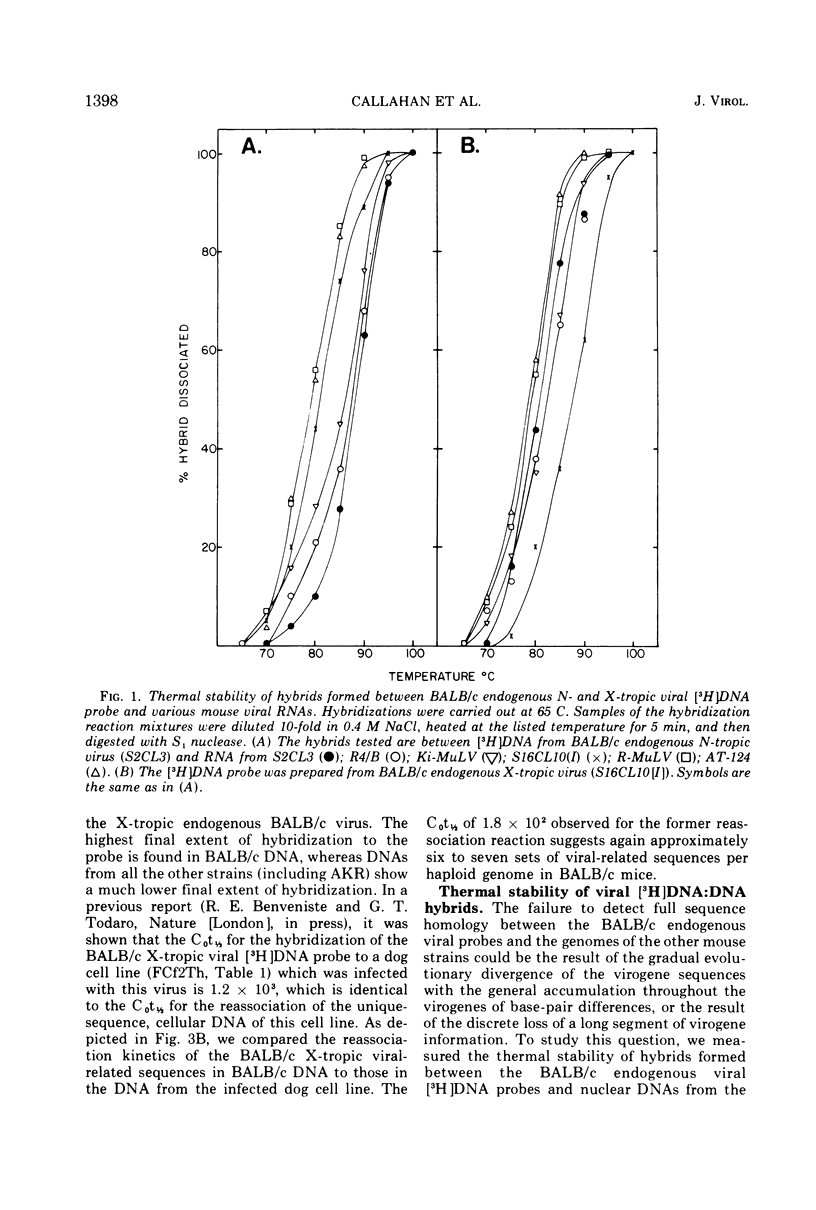
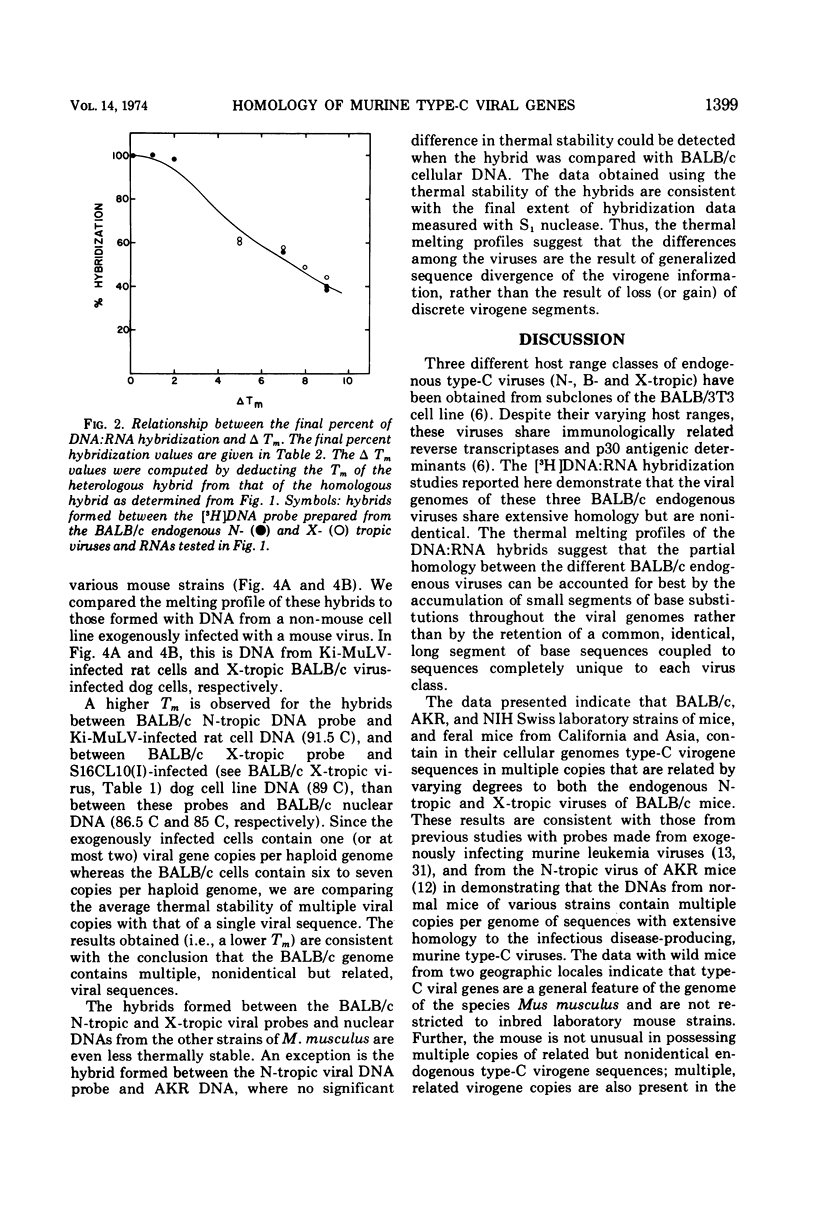
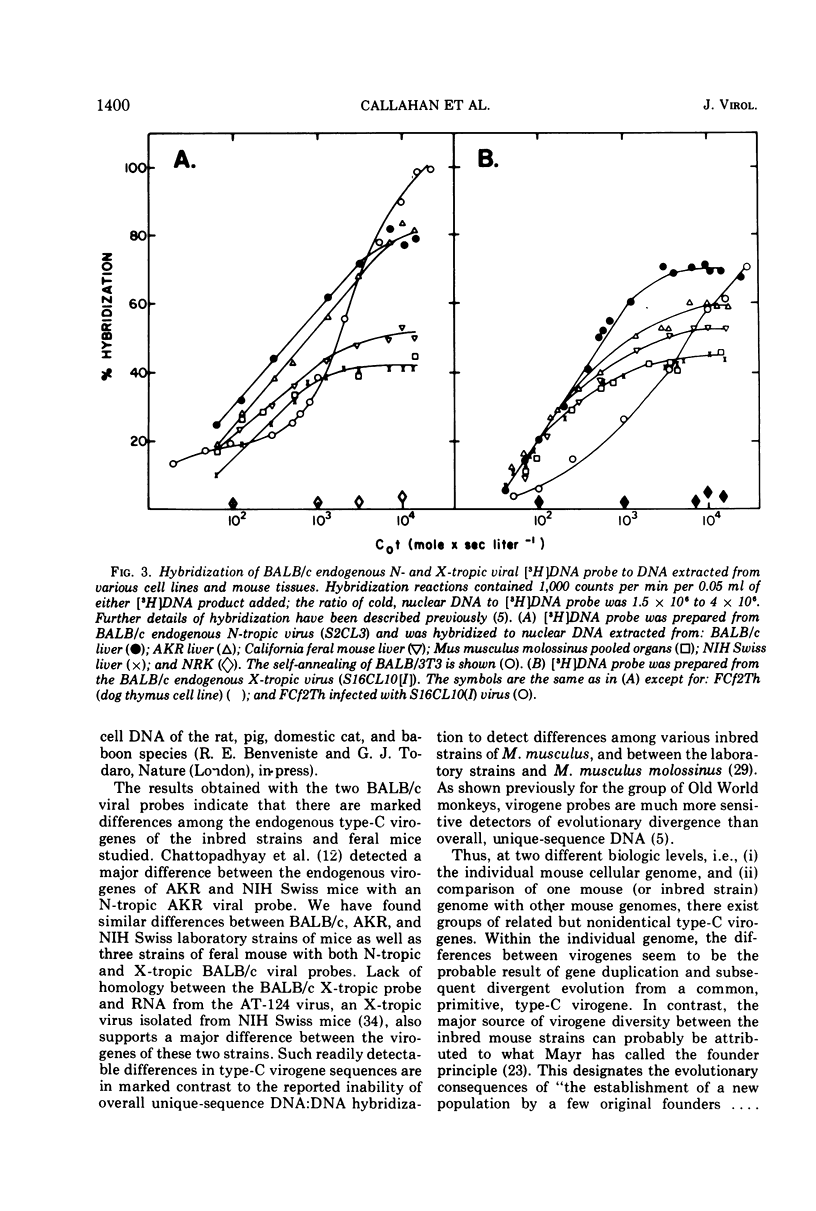
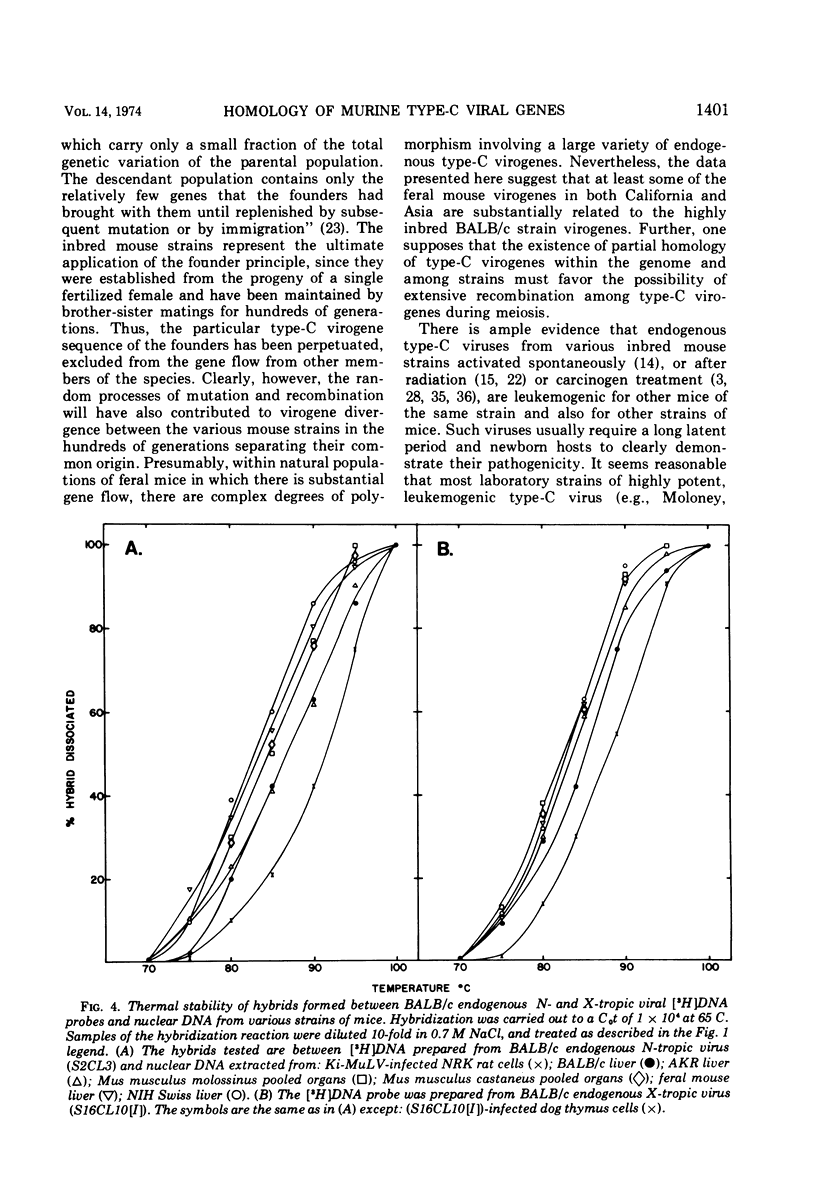
The nucleic acid sequence homology between various murine, endogenous type-C viruses (three host range classes of BALB/c virus, the AT-124 virus, and the CCL 52 virus) and two laboratory strains of murine leukemia virus (Rauscher and Kirsten) was determined by DNA:RNA hybridization. The viral sequences exhibit varying degrees of partial homology. DNA:DNA hybridizations were performed between [3H]DNA probes prepared from N- and X-tropic BALB/c endogenous viruses and cellular DNAs from BALB/c, NIH Swiss, and AKR inbred mouse strains as well as from California feral mice and the Asian mouse subspecies Mus musculus molossinus and M. musculus castaneus. All of these strains of mice are shown to possess multiple (six to seven per haploid genome), partially related copies of type-C virogenes in their DNAs. Thermal melting profiles of the DNA:RNA and DNA:DNA hybrids suggest that the partial homology of the viral nucleic acid sequences is the result of base alterations throughout the viral genomes, rather than the loss of discrete segments of viral sequences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Ball J. K., McCarter J. A. Repeated demonstration of a mouse leukemia virus after treatment with chemical carcinogens. J Natl Cancer Inst. 1971 Apr;46(4):751–762. [PubMed] [Google Scholar]
- Barski G., Blanchard M. G., Youn J. K., Leon B. Expression of malignancy in interspecies Chinese hamster X mouse cell hybrids. J Natl Cancer Inst. 1973 Sep;51(3):781–792. doi: 10.1093/jnci/51.3.781. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Todaro G. J. A distinct class of inducible murine type-C viruses that replicates in the rabbit SIRC cell line. Proc Natl Acad Sci U S A. 1974 Mar;71(3):602–606. doi: 10.1073/pnas.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS L. "Spontaneous" leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc Soc Exp Biol Med. 1951 Jan;76(1):27–32. [PubMed] [Google Scholar]
- GROSS L. [Serial cell-free passage of a radiation-activated mouse leukemia agent]. Proc Soc Exp Biol Med. 1959 Jan;100(1):102–105. doi: 10.3181/00379727-100-24538. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. VI. The extent of base sequence divergence among the DNAs of various rodents. Biochem Genet. 1970 Jun;4(3):425–446. doi: 10.1007/BF00485758. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Hartley J. W., Spahn G. J., Rabstein L. S., Whitmire C. E., Turner H. C., Huebner R. J. Prevalence of the group-specific (gs) antigen and infectious virus expressions of the murine C-type RNA viruses during the life span of BALB-cCr mice. Int J Cancer. 1972 Sep 15;10(2):283–289. doi: 10.1002/ijc.2910100208. [DOI] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Ribacchi R., Giraldo G. Leukemia virus release in chemically or physically induced lymphomas in BALB-c mice. Natl Cancer Inst Monogr. 1966 Sep;22:701–711. [PubMed] [Google Scholar]
- Rice N. R., Straus N. A. Relatedness of mouse satellite deoxyribonucleic acid to deoxyribonucleic acid of various Mus species. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3546–3550. doi: 10.1073/pnas.70.12.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springgate C. F., Battula N., Loeb L. A. Infidelity of DNA synthesis by reverse transcriptase. Biochem Biophys Res Commun. 1973 May 15;52(2):401–406. doi: 10.1016/0006-291x(73)90725-0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Goodman N. C., Cho J. R., Ruprecht R. M., Redfield R. R., Spiegelman S. The presence of unique DNA sequences after viral induction of leukemia in mice. (RNA tumor virus-nucleic acid hybridization-insertion of viral DNA). Proc Natl Acad Sci U S A. 1974 May;71(5):1705–1709. doi: 10.1073/pnas.71.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz Y., Lennette E. H., Oshiro L. S., Cremer N. E. Release of C-type particles from normal rat thymus cultures and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1971 Jan;46(1):11–23. [PubMed] [Google Scholar]
- Todaro G. J., Arnstein P., Parks W. P., Lennette E. H., Huebner R. J. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc Natl Acad Sci U S A. 1973 Mar;70(3):859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J. Spontaneous release of type C viruses from clonal lines of spontaneously transformed Blab-3T3 cells. Nat New Biol. 1972 Nov 29;240(100):157–160. doi: 10.1038/newbio240157a0. [DOI] [PubMed] [Google Scholar]


