Table 1.
Synthesis and isolation of allylboronic acids.[a]
| Entry | Substrate | Cat.[b] (mol %) | Solvent[c] (molarity) | t [h] | Product | Yield[d] [%] |
|---|---|---|---|---|---|---|
| 1 | 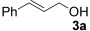 |
2 a (0.5) | MeOH (1.0) | 18 | 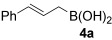 |
61[e] |
| 2 | 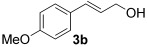 |
2 a (0.5) | MeOH (1.0) | 0.2 | 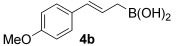 |
80 |
| 3 | 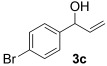 |
2 a (0.2) | MeOH (1.0) | 2 | 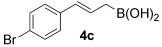 |
71 |
| 4 | 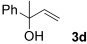 |
2 a (0.2) | DMSO/H2O 3:2 (1.0) | 14 | 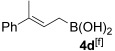 |
55 |
| 5 | 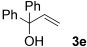 |
2 a (2.0) | DMSO/H2O 4:1 (1.0) | 13 | 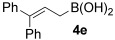 |
71 |
| 6 | 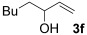 |
2 a (0.2) | MeOH (1.0) | 1[g] | 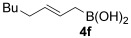 |
51 |
| 7 | 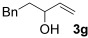 |
2 a (0.3) | MeOH (1.0) | 1[g] | 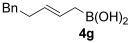 |
50 |
| 8 | 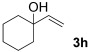 |
2 a (0.5) | DMSO/H2O 3:1 (1.0) | 18 | 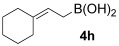 |
68 |
| 9 | 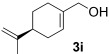 |
2 b (5.0) | DMSO/H2O 9:1 (0.4) | 0.2 | 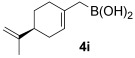 |
67 |
| 10 | 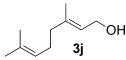 |
2 a (5.0) | DMSO/H2O 4:1 (0.5) | 18 | 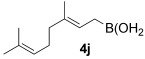 |
77[h] |
| 11 | 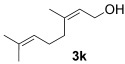 |
2 a (5.0) | DMSO/H2O 4:1 (0.5) | 18 | 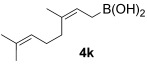 |
79[h] |
| 12 | 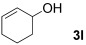 |
2 b (5.0) | DMSO/H2O 9:1 (1.0) | 1 | 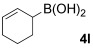 |
25 |
[a] Unless otherwise stated, Pd-catalyst 2 a or 2 b (0.2–5 mol %) and diboronic acid 1 (2.4 mmol) were added to allylic alcohol 3 (2 mmol) in the given solvent. After filtration, 4 was precipitated with degassed brine. [b] Catalyst loading (mol %) is given in parentheses. [c] The molar concentration of 3 in the given solvent is in parenthesis. [d] Yield of isolated product. [e] 65 % yield was obtained when the reaction was performed on a gram scale using 6 mmol of 3 a. [f] 5:1 E/Z ratio. [g] The reactions were performed at 0 °C. [h] The product was isolated by extraction. The yield was determined by 1H NMR spectroscopy using naphthalene as an internal standard.
