Abstract
Background: The effect of corticosteroid therapy on corneal wound healing is controversial. The objective of this study was to evaluate the effects of combination therapy with dexamethasone and acetylcysteine at different times and durations on experimentally-induced corneal wounds and haze in rabbits.
Methods: Eighteen adult New Zealand white rabbits were divided into three groups of six each. Under anesthesia corneal wounds were created surgically in the center of all eyes. The right eyes of rabbits in group 1 were treated topically with acetylcysteine and dexamethasone immediately after surgery, those in group 2 were treated with acetylcysteine from day 1 and with acetylcysteine and dexamethasone from day 8, and those in group 3 were treated with acetylcysteine from day 1 and with acetylcysteine and dexamethasone from day 15. The left eyes were assigned as controls and were treated with normal saline. All eyes were treated six times a day for 28 days. Corneal wounds were measured by fluorescein staining every day.
Results: The combination of acetylcysteine and dexamethasone in group 1 significantly increased mean healing time, but did not change that in groups 2 and 3. Clinical and histopathologic examinations revealed that one month after the ulceration in groups 1 corneal haze was greater in treated than in the control eyes. Moreover, there was no significant difference between the control and treated eyes of group 1, 2, or 3 in terms of corneal haze at two or three months after the ulceration.
Conclusions: The findings of the present study show that the association of 3% concentration of NAC and 0.1% concentration of dexamethasone immediately after corneal ulceration can delay corneal wound healing, and consequently produce more corneal haze. Thus, the use of 0.1% concentration of dexamethasone should be delayed at least until the completion of the epithelial defects.
Key Words: Acetylcysteine, dexamethasone, rabbits, wound healing, corneal wounds
Introduction
Following ocular surgery or trauma, the majority of patients develop some degree of loss of corneal transparency, namely haze.1 Scar tissue is created due to loss of the regulation of the biosynthesis of macromolecules of the corneal extracellular matrix as well as increased degradation of the corneal matrix by neutral endopeptidases, especially matrix metalloproteinase (MMP)-9.2-5
Much research has been done to examine ways to improve corneal wound healing and to decrease corneal haze after photo refractive keratectomy (PRK) or traumatic corneal ulcers.6-10 Collagenase inhibitors are reported to be the most effective drugs. These compounds exert their functions by inhibiting the removal and replacement phases in stromal repair.7 Steroids have also been used to reduce corneal haze. Although patients are usually treated prophylactically with topical steroids, their effectiveness in reducing haze and regression remain controversial.11-16
We have already shown that using a collagenase inhibitor, acetylcysteine, in combination with a steroid (dexamethasone) from the first day after corneal ulceration could delay the wound healing, but did not decrease corneal haze compared with no treatment.17 We hypothesized that the use of this combination at different times can lead to beneficial results. Therefore, the present study was designed to evaluate whether simultaneous use of acetylcystein and dexamethasone at different times would accelerate corneal wound healing and decrease corneal haze in experimentally induced corneal ulceration in rabbits' eyes.
Material and Methods
Eighteen adult New Zealand white rabbits were selected. All rabbits, weighing 1.05 to 1.75 kg (mean±SD, 1.28±0.20 kg), were clinically healthy. All animal procedures were approved by the Institutional Committee for Animal Care and Use. The animals were housed under light controlled at a dim level for comfort.
The rabbits were anesthetized with an intramuscular injection of a solution containing ketamine hydrochloride (35 mg/kg), xylazine hydrochloride (5 mg/kg) and acepromazine maleate (1 mg/kg). A pediatric eyelid speculum was placed under the lids to open the eyelids and expose the cornea. Then, the inferior rectus muscle was grasped by a muscle hook to control the ocular movements during trephination. A 6.5 mm calibrated corneal trephine was used to induce an ulcer in the center of the cornea. The trephine depth was set to expose about the anterior one-third of the cornea (100 microns). The trephinated area was separated from the cornea by a crescent bevel-up blade. This procedure was performed on both eyes of all rabbits.
The rabbits were divided into three groups of six animals each. All rabbits were administered one drop of gentamicin ophthalmic solution topically in both eyes at least five minutes before receiving drugs (acetylcystein or dexamethasone) or placebo. Gentamicin treatment started immediately after surgery and continued for five days to prevent infection. The right eye was elected to receive treatment, while the left eye was assigned to receive placebo. The right eyes of rabbits in group 1 received a topical application of one drop of 3% acetylcysteine (Rotexmedica, Germany) and one drop of 0.1% dexamethasone (Sinadarou, Tehran, Iran) starting immediately after surgery. In groups 2, the right eyes received two drops of 3% acetylcysteine from day 1 (for one week), and one drop of 3% acetylcysteine and one drop of 0.1% dexamethasone from day 8 (beginning of week 2). In group 3, the right eyes were treated with two drops of 3% acetylcysteine from day 1 (for two weeks), and with one drop of 3% acetylcysteine and one drop of 0.1% dexamethasone from day 15 (beginning of week 3). Control eyes in all groups received two drops of isotonic saline (NaCl 0.9%). All eyes were treated six times per day (once every two hours from 8 am to 6 pm) for 28 days.
The wounds were stained by fluorescein at 4 pm every day in order to record by photography, then the defect area (in mm2) was calculated using the AutoCAD 2005 program. Healing was considered to be complete when no fluorescein stain uptake was visible with a cobalt light source. All eyes were thoroughly examined to assess the corneal haze at two and three months after the beginning of surgery. The daily healing was calculated for each eye using the following formula:
[(Wound area on each day – wound area on the next day)/ wound area on that day]×100
Corneal haze was categorized as cornea without opacity, mild opacity, moderate opacity, or severe opacity based on light transmission and visibility of the fundus by the same examiner throughout the study.
Every month for three months from the beginning of the study two rabbits in each group were deeply anesthetized and both of their eyes were enucleated. The eyes were then immersed immediately in 10% buffered formalin, processed, and sectioned with a microtome at five µm thickness. The sections were stained with hematoxylin and eosin, and examined by light microscopy. They were examined for the epithelial changes (hypertrophy and hyperplasia), noevascularizations, stromal irregularity and edema, scarring of stroma and cellular changes.
Statistical Analysis
The mean daily healing against time (days) were fitted to a linear regression equation and slope of healing curves were calculated for each group. The slopes of healing for the control (those receiving saline) and treated (those receiving drugs) eyes of each group was compared using Wilcoxon signed ranks test. Similar test was also used to compare total healing time or corneal haze in the control and treated eyes of each of the three groups.18
Results
Macroscopic Evaluation
Analysis of 36 healing curves and their slopes revealed that the mean healing time or mean daily healing between of control and treatment eyes in group 1 (NAC+Dexa1) was significant (table 1 and figures 1-3). These findings show that drug combinations increased the mean healing time and decreased mean daily healing compared with the control eyes (P<0.05). However, mean healing time or mean daily healing of the control and treatment eyes in group 2 (NAC+Dexa8) or group 3 (NAC+Dexa15) was not significant. This means that these two groups did not exhibit the delayed wound healing observed in group 1.
Table 1.
The values (means±SD, n=6) of mean healing time, mean daily healing, and slopes of healing curves in the control and treated eyes of group 1 receiving N-acetylcystein and dexamethasone (NAC+dexa1), group 2 receiving N-acetylcystein from day 1 and dexamethasone from day 8 (NAC+dexa8) and group 3 receiving N-acetylcystein from day 1 and dexamethasone from day 15 (NAC+dexa15)
| Variables |
Group 1
(NAC+Dexa1) |
Group 2
(NAC+Dexa8) |
Group 3
(NAC+Dexa15) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | P values | Control | Treatment | P values | Control | Treatment | P values | |
| Healing time (days) | 8.2±1.9 | 16.0±6.7a | 0.042 | 8.2±1.8 | 9.0±3.0 | 0.680 | 7.8±2.5 | 7.7±2.0 | 0.655 |
| Daily healing (%) |
12.8±2.9 | 7.1±2.4a | 0.043 | 12.8±2.8 | 12.4±4.8 | 0.786 | 13.8±4.1 | 13.9±3.9 | 0.655 |
| Slopes of healing curves | -0.211±0.030 | -0.455±0.199a | 0.028 | -0.204± 0.313 |
-0.227± 0.088 |
0.600 | -0.201± 0.685 |
-0.187± 0.038 |
0.753 |
a indicates significant difference from control eyes.
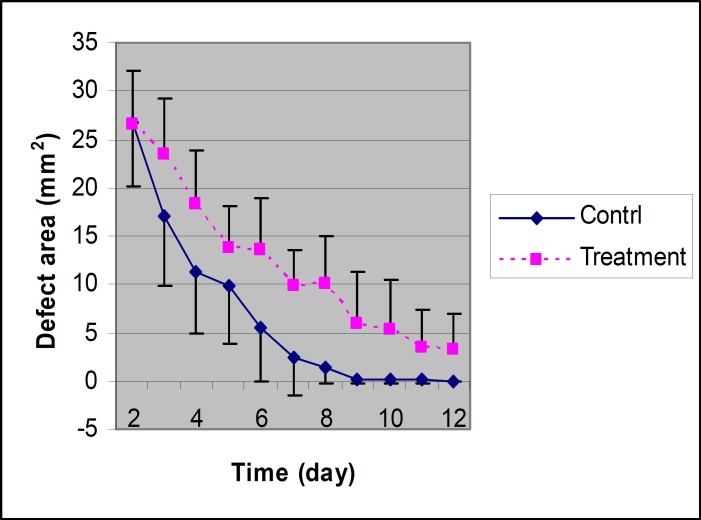
Figure 1.
The areas of epithelial defect in treated and control eyes at different times in rabbits of group 1 (n=6). The stops of treated eyes is significantly different compared with control eyes (P=0.028).
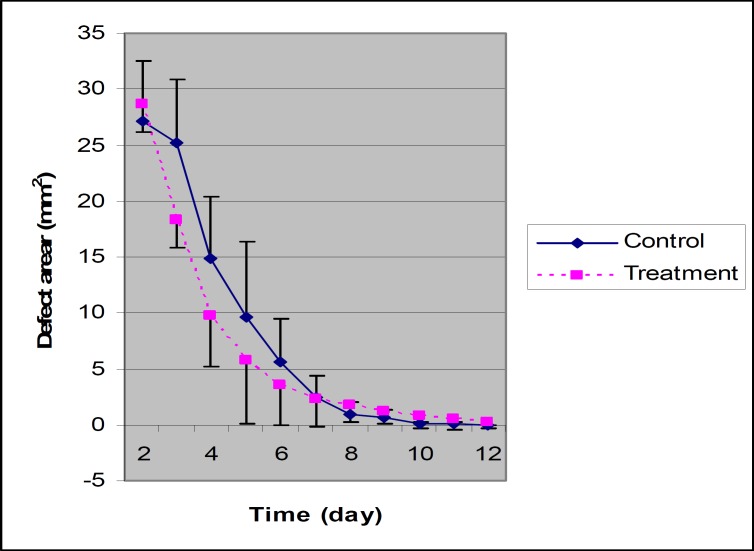
Figure 2.
The areas of epithelial defect in treated and control eyes at different times in rabbits of group 2 (n=6). There are no difference between treated and control eyes.
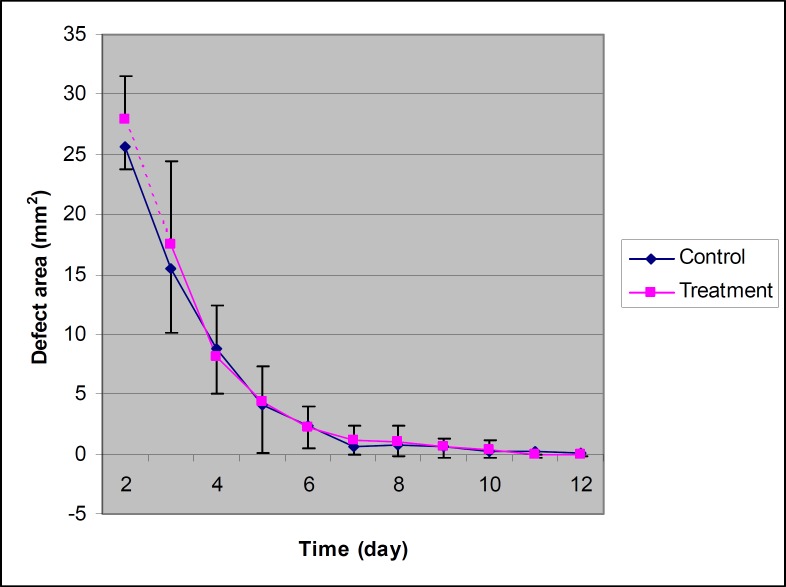
Figure 3.
The areas of epithelial defect in treated and control eyes at different times in rabbits of group 3 (n=6). There are no difference between treated and control eyes.
On the first day after the operations all rabbits showed conjunctival hyperemia. Moreover, blepharospasm and photophobia were seen during the first two days after the operations, therefore, the animals were kept in a relatively dark room, and the lights were turned on only at the time of examinations and treatments.
In the first week after the operations four rabbits showed conjunctival infection (treatment eye of rabbit 6 in group 1, control eyes of rabbits 14, 16 and 17 in group 3), however, they were cured after a few days without any complications. Ophthalmic examination at one month after the operation showed that in group 1 corneal haze in treated eyes was insignificantly greater than that in the control group, whereas the examination at two and three months after the operations revealed that corneal haze in treated eyes was insignificantly less than that in control eyes. The treated eyes of rabbits in groups 2 and 3 showed insignificantly lesser corneal haze that the relevant control eyes at one, two and three months after the operation. Three months after the operation both control and treated eyes in groups 1, 2, or 3 had corneal haze, and the opacity of the cornea around the ulcer was more severe than in the center.
Histopathologic Examination
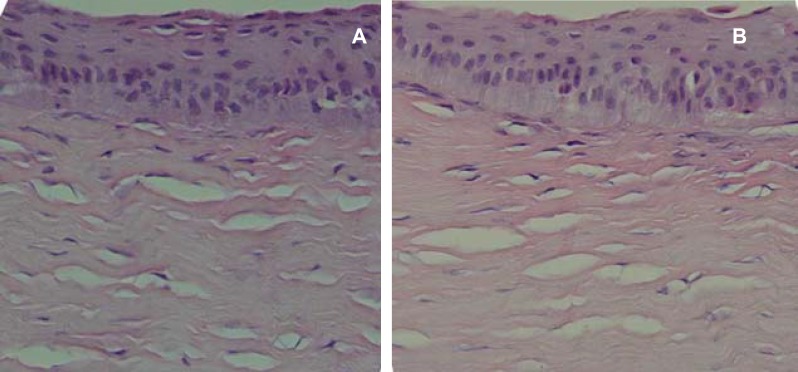
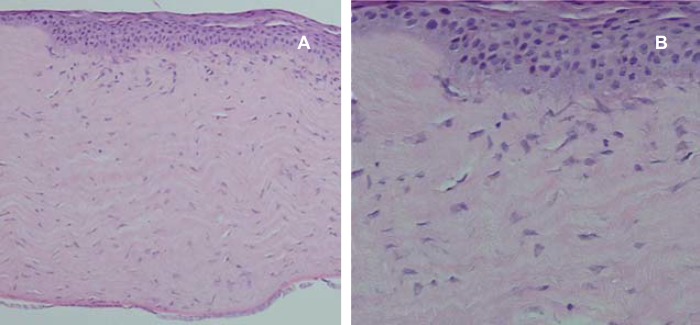
Histopathologic examination of cornealsamples revealed that all flaps had been taken from the anterior one-third of the corneal stroma. Few samples showed epithelial hypertrophy and hyperplasia, but no difference was detected between control and treated eyes (figures 4 and 5). The stroma seemed variably edematous in 28% of control eyes, while edema was less remarkable in treated eyes (figure 5). One of the control eyes in group 3 showed stromal neovascularization.
Figure 4.
Histology of the cornea in a control (a) and treatment eye (b) in group 2 one month after surgery showing some epithelial hyperplasia. No difference was detected between the control and treatment eyes (H&E, ×400).
Figure 5.
Histology of the cornea in a control eye in group 3 one month after surgery showing epithelial hyperplasia and superficial stromal irregularity and edema (H&E a: ×100, b: ×400).
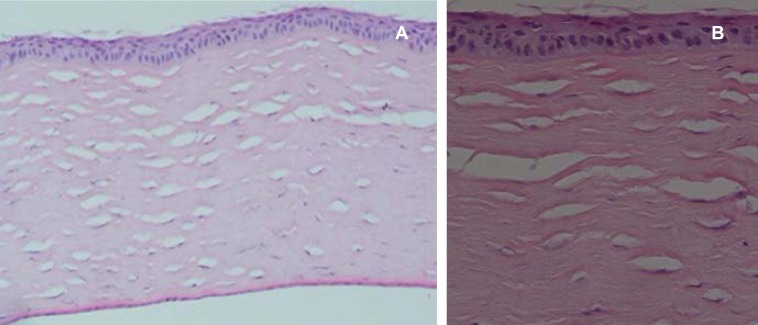
The stromal lamellae were arranged more regularly in both the control and treated eyes over time. They were arranged more regularly in the control than in the treated eyes of group 1, but not those of groups 2 or 3 (figure 6). Stromal scarring in the second and third months was similar in both the control and treated eyes of groups 2 or 3. After three months, the epithelium thickness was normal and edema disappeared in all eyes except a control one. Descemet’s membrane and endothelium were normal in all eyes. Therefore, it seems that dexamethsone treatment resulted in less edema in the early stages, and less pronounced scarring in later stages.
Figure 6.
Histology of the cornea in a treated eye three months after surgery. The epithelium is relatively normal with minimal scarring in the stroma (H&E a: ×100, b: ×400).
Discussion
In corneal wounds and after keratorefractive surgeries, even in cases in which healing is eventually accomplished, opacity of the cornea and regression occur as a result of scarring.2,3 To improve the results of corneal surgeries, attention has been focused on modulating the postoperative wound healing process.19,20
Both haze and regression arise from an overly aggressive wound healing response to surgery. The healing response involves three processes including removal, repair and replacement. Initially, there is the breakdown and removal of debris from the margins of the wound. This is then followed by the repair of the damaged structures and the replacement of irreparable parts. In tissues with a good potential for regeneration, such as the epithelium, replacement is by structures similar to those lost.21 On the contrary, where the regeneration potential is not well enough, as in the stroma, alternative tissue components are produced in the form of a scar, and these often have characteristics that differ from those of the original tissue.22 Following conventional surgery and laser photoablation, replacement occurs by the migration and division of keratocytes, and the synthesis of extracellular material. These components are largely responsible for corneal haze and compromised visual function.23
Several authors have reported that low concentrations of NAC, if used topically, did have a beneficial effect on corneal wound healing.6,7,24-33 We have already shown that topical application of 3% NAC in dogs did improve would healing.33 We have also shown that the topical use of 3% concentration of NAC and 0.1% concentration of dexamethasone delayed the corneal wound healing in rabbits, and did not decrease corneal haze compared to the control groups.17 This shows that dexamethasone, but not acetylcysteine, delays corneal wound healing.
In the present study, the combination of 3% concentration of NAC and 0.1% dexamethasone in group 1, in which treatment started immediately after ulcerations, delayed corneal wound healing. In group 2, where dexamethasone treatment started on day 8 after the operation, a small non-significant delay occurred in the wound healing, because the epithelial defects had not been completed. In group 3, treatment with dexamethasone was started on day 15 after the operation, when epithelial healing had been completed in some eyes, therefore, the combination therapy caused a partial non-significant acceleration of wound healing. However, it seemed that dexamethasone still prevented the acceleration effect of acetylcysteine on wound healing in those eyes, where the re-epithelialization of the epithelium was not completed. Since the changes in healing in groups 2 and 3 were not significant, more studies are needed to confirm these results.
Ophthalmologic examinations showed that one month after the operations in group 1 corneal haze of treated eyes was greater than that in the control eyes. By contrast, it was less in treated eyes than in the control eyes in group 2 and 3. Although not significant, two and three months after the operation in all groups, corneal haze in treated eyes of groups 1, 2, or 3 was less than that in respective controls. These results show that using dexamethasone immediately after corneal ulceration can delay wound healing and increase corneal haze. However, when dexamethasone is used after the completion the epithelial defect or a few days later, it cannot delay wound healing and may decrease corneal haze.
The use of local corticosteroids in the management of corneal wound healing is controversial.11-16,34-39 Some investigators have reported that dexamethasone have some beneficial effects on corneal wound healing.11-16 On the contrary, others have shown that corticosteroids, including dexamethasone, are associated with an unacceptably high incidence of unwanted side effects.34-39
Francois and Feher reported that corticosteroid treatment was harmful during a critical period of two to three weeks after the burn, because steroids could retard the fibroblastic repopulation of the acellular stroma during this period.37 Such a retardation decreases the synthesis of new collagen in the wound, and results in more severe corneal ulceration. After such a period, the repopulation of fibroblasts occurs in the stroma, and stromal matrix materials are properly secreted. Therefore, corticosteroid treatment is less harmful.
Kim et al. showed that an increase in the number of keratocytes and degree of apoptosis could increase the corneal gaze.10 Epithelial defects are known to induce keratocyte apoptosis and an inflammatory cell response, producing alterations in the extracellular stromal matrix composition that are directly proportional to the healing time of the epithelial defects. 9 Faster epithelial healing induces less keratocyte apoptosis and inflammatory cell infiltration, and reduces the upregulation of chondroitin sulfate in the corneal stroma adjacent to the epithelial defect. Minimizing stromal changes by inducing faster epithelial healing can improve the refractive outcomes.9
In the present study, the wounds were created in both the epithelium and stroma. Therefore, in group 1 it seems that dexamethasone delayed the re-epithelialization of the epithelium, induced keratocyte apoptosis and the upregulation of extracellular materials in the corneal stroma adjacent to the epithelial defect, and increased stromal changes and corneal haze. On the contrary, in group 3 corneal haze decreased compared with controls.
Conclusion
The findings of the present study shows that the association of 3% concentration of NAC and 0.1% concentration of dexamethasone immediately after corneal ulceration can delay corneal wound healing, and consequently produce more corneal haze. Thus, the use of 0.1% concentration of dexamethasone should be delayed at least until the completion of the epithelial defects. Further studies with larger sample size are needed to confirm the findings of this study.
Acknowledgment
This study was supported by a research grant (84-VE-1781-C308) from the Research Council of Shiraz University. The authors would like to thank Dr Maryam Ansari for her assistance with the statistical analysis of the findings.
Conflict of Interest: None declared
References
- 1.Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit cornea. A biphasic process. Invest Ophthalmol Vis Sci. 1986;27:464–73. [PubMed] [Google Scholar]
- 2.Kubota M, Fagerholm P. Corneal alkali burn in the rabbit. Water balance, healing and transparency. Acta Ophthalmol (Copenh) 1991;69:635–40. doi: 10.1111/j.1755-3768.1991.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Saito J, Yanai R, et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22:113–33. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara M, Girard MT, Kublin CL, et al. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodeling cornea. Dev Biol. 1991;147:425–39. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- 5.Twining SS, Schulte DP, Zhou X, et al. Changes in rat corneal matrix metalloproteinases and serine proteinases under vitamin A deficiency. Curr Eye Res. 1997;16:158–65. doi: 10.1076/ceyr.16.2.158.5085. [DOI] [PubMed] [Google Scholar]
- 6.Petroutsos G, Guimaraes R, Giraud JP, et al. Effect of acetylcysteine (Mucomyst) on epithelial wound healing. Ophthalmic Res. 1982;14:241–8. doi: 10.1159/000265198. [DOI] [PubMed] [Google Scholar]
- 7.Corbett MC, O'Brart DP, Patmore AL, Marshall J. Effect of collagenase inhibitors on corneal haze after PRK. Exp Eye Res. 2001;72:253–9. doi: 10.1006/exer.2000.0959. [DOI] [PubMed] [Google Scholar]
- 8.Stiles J, Honda CN, Krohne SG, Kazacos EA. Effect of topical administration of 1% morphine sulfate solution on signs of pain and corneal wound healing in dogs. Am J Vet Res. 2003;47:813–18. doi: 10.2460/ajvr.2003.64.813. [DOI] [PubMed] [Google Scholar]
- 9.Esquenazi S, He J, Bazan HEP, Bazan NG. Use of autologous serum in corneal epithelial defects post-lamellar surgery. Cornea. 2005;24:992–7. doi: 10.1097/01.ico.0000160967.65953.ea. [DOI] [PubMed] [Google Scholar]
- 10.Kim TI, Lee SY, Pak JH, et al. Mitomycin C, ceramide, and 5-fluorouracil inhibit corneal haze and apoptosis after PRK. Cornea. 2006;25:55–60. doi: 10.1097/01.ico.0000167878.11687.9a. [DOI] [PubMed] [Google Scholar]
- 11.Hoffart L, Matonti F, Conrath J, et al. Inhibition of corneal neovascularization after alkali burn: comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Experiment Ophthalmol. 2010;38:346–52. doi: 10.1111/j.1442-9071.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang SW, Chou SF, Yu SY. Dexamethasone reduces mitomycin C-related inflammatory cytokine expression without inducing further cell death in corneal fibroblasts. Wound Repair Regen. 2010;18:59–69. doi: 10.1111/j.1524-475X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Park SC, Kim JH. Effect of steroids and nonsteroidal anti-inflammatory agents on stromal wound healing following excimer laser keratectomy in rabbits. Ophthalmic Surg Lasers. 1996;27:S481–6. [PubMed] [Google Scholar]
- 14.Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001;79:23–7. doi: 10.1034/j.1600-0420.2001.079001023.x. [DOI] [PubMed] [Google Scholar]
- 15.Tani E, Katakami C, Negi A. Effects of various eye drops on corneal wound healing after superficial keratectomy in rabbits. Jpn J Ophthalmol. 2002;46:488–95. doi: 10.1016/s0021-5155(02)00541-5. [DOI] [PubMed] [Google Scholar]
- 16.Yülek F, Ozdek S, Gürelik G, Hasanreisoğlu B. Effect of topical steroids on corneal epithelial healing after vitreoretinal surgery. Acta Ophthalmol Scand. 2006;84:319–22. doi: 10.1111/j.1600-0420.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 17.Sarchahi AA, Maimandi A, Amani M. Effects of acetylcysteine and dexamethasone on experimental corneal wounds in rabbits. Ophthalmic Res. 2008;40:41–8. doi: 10.1159/000111158. [DOI] [PubMed] [Google Scholar]
- 18.Armitage P, Berry G, Mathews JNS. Statistical methods in medical research. 4th ed. Blackwell Science; 324 pp. [Google Scholar]
- 19.Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290:S12–23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- 20.Clark A. New discoveries on the roles of matrix metalloproteinases in ocular cell biology and pathology. Invest Ophthalmol Vis Sci. 1998;39:2514–6. [PubMed] [Google Scholar]
- 21.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–8. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cintron C, Convington HI, Kublin CL. Morphogenic analysis of proteoglycans in rabbit corneal scars. Invest Ophthalmol Vis Sci. 1990;31:1789–98. [PubMed] [Google Scholar]
- 23.Tuft SJ, Zabel RW, Marshall J. Corneal repair following keratectomy: a comparison between conventional surgery and laser photoablation. Invest Ophthalmol Vis Sci. 1989;30:1769–77. [PubMed] [Google Scholar]
- 24.Absolon MJ, Brown CA. Acetylcysteine in keratoconjunctivitis sicca. Br J Ophthalmol. 1968;52:310–16. doi: 10.1136/bjo.52.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urgancioglu B, Bilgihan K, Engin D, et al. Topical N-acetylcysteine reduces interleukin-1-alpha in tear fluid after laser subepithelial keratectomy. Eur J Ophthalmol. 2009;19:554–9. doi: 10.1177/112067210901900406. [DOI] [PubMed] [Google Scholar]
- 26.Scorolli L, Meduri A, Morara M, et al. Effect of cysteine in transgenic mice on healing of corneal epithelium after excimer laser photoablation. Ophthalmologica. 2008;222:380–5. doi: 10.1159/000151691. [DOI] [PubMed] [Google Scholar]
- 27.Kubota M, Shimmura S, Kubota S, et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. 2011;52:427–33. doi: 10.1167/iovs.10-6167. [DOI] [PubMed] [Google Scholar]
- 28.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali- burned rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1569–75. [PubMed] [Google Scholar]
- 29.Kanao S, Kouzuki S, Isuneno M, et al. Clinical applicatin of 3% N-acetylcysteine eye drops in corneal diseases in dogs. J Japan vet Med Ass. 1993;46:488–91. [Google Scholar]
- 30.Marsh RJ, Cooper M. Ophthalmic zoster: Mucous plaque keratitis. Br J Ophthalmol. 1987;71:725–8. doi: 10.1136/bjo.71.10.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thermes F, Molon-Noblot S, Grove J. Effects of acetylcysteine on rabbit conjunctival and corneal surfaces. Invest Ophthalmol Vis Sci. 1991;32:2958–63. [PubMed] [Google Scholar]
- 32.Haut J, Labrune P, Ullern M. New trial treatment of dry eye with acetylcysteine ophthalmic solution. Bull Soc Ophtalmol Fr. 1977;77:165–7. [PubMed] [Google Scholar]
- 33.Aldavood SJ, Behyar R, Sarchahi AA, et al. Effect of acetylcysteine on experimental corneal wounds in dogs. Ophthalmic Res. 2003;35:319–23. doi: 10.1159/000074070. [DOI] [PubMed] [Google Scholar]
- 34.Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y. Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol. 1982;66:705–8. doi: 10.1136/bjo.66.11.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SE, Mohan RR, Mohan RR, et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Kurosaka D, Yoshino M, et al. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2002;43:2603–8. [PubMed] [Google Scholar]
- 37.Francois J, Feher J. Studies with the polarization microscope of the fibroblastic activity of the regenerating rabbits cornea under the influence of corticosteroids. Exp Eye Res. 1973;15:201–5. doi: 10.1016/0014-4835(73)90214-5. [DOI] [PubMed] [Google Scholar]
- 38.Corbett MC, O'Brart DPS, Marshall J. Do topical corticosteroids have a role following excimer laser photorefractive keratectomy? J Refract Surg. 1995;11:380–7. doi: 10.3928/1081-597X-19950901-15. [DOI] [PubMed] [Google Scholar]
- 39.Chung JH, Kang YG, Kim Hj. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: morphological changes during the repair process. Graefes Arch Clin Exp Ophthalmol. 1998;236:537–45. doi: 10.1007/s004170050118. [DOI] [PubMed] [Google Scholar]