Figure 4.
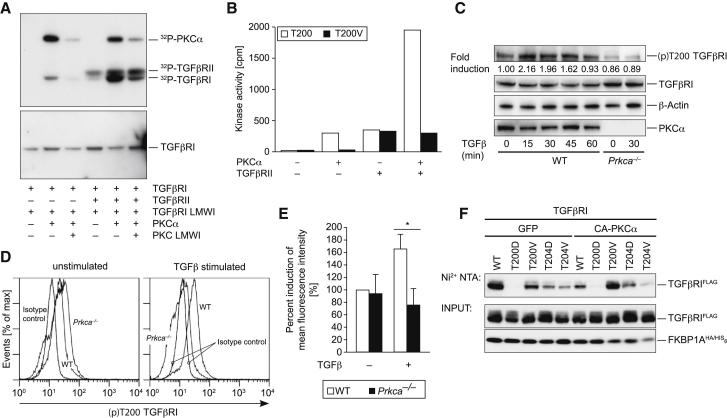
PKCα Phosphorylates T200 on TGFβRI
(A) Kinase assays of the glutathione S-transferase (GST) fusion protein of the cytoplasmic subdomain of TGFβRI (amino acids 148–503) were incubated with recombinant PKCα and TGFβRII as indicated. Phosphorylation was detected by autoradiography. The GST-antibody immunoblot (lower panel) confirmed equal loading.
(B) The phosphorylation rates of T200WT and T200V mutant motifs were measured by the incorporation of 32Pi from γ32P-ATP incubated with PKCα and/or TGFβRII kinases. The following abbreviation is used: cpm, counts per minute.
(C) Naive CD4+ T cells were stimulated with TGFβ as indicated. TGFβ-inducible phosphorylation of T200 on TGFβRI was observed in WT cells, but not Prkca−/− cells, as shown by a representative IB.
(D and E) The intracellular induction by (p)T200 on TGFβRI with the (p)T200-specific antibody and the quantification of the percent induction of MFI are shown. In (E), the error bars represent ± SEM. ∗p < 0.05.
(F) Cotransfection of Jurkat T cells with constitutively active A25E PKCα significantly diminished binding of FKBP1A to the WT TGFβRI, but not the neutral-exchange T200V mutant of TGFβRI. Representative data were derived from at least two independent experiments.
(See also Figure S4).