Figure 2.
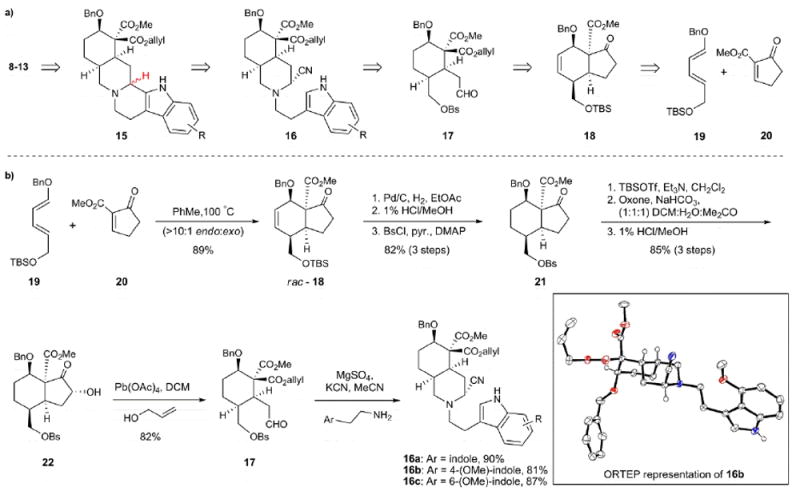
Retrosynthetic plan and synthesis of the aminonitrile Pictet-Spengler substrates. a) A variety of yohimbinoid natural products can be accessed from common hydrindanone precursor 18. b) The Diels-Alder cyclization between diene 19 and dienophile 20 provides hydrindanone 18 setting four contiguous stereocenters. Conversion of hydrindanone 18 into aldehyde 17 sets the stage for rapid formation of aminonitriles (16a-c) with varying substitution on the indole moiety. Bs, benzenesulfonyl; TBS, tert-butyldimethylsilyl; Bn, benzyl; PhMe, toluene; pyr, pyridine; DMAP, 4-dimethylaminopyridine; TBSOTf, tert-butyldimethylsilyl triflate; Oxone, potassium monopersulfate; DCM, dichloromethane.
