Figure 4.
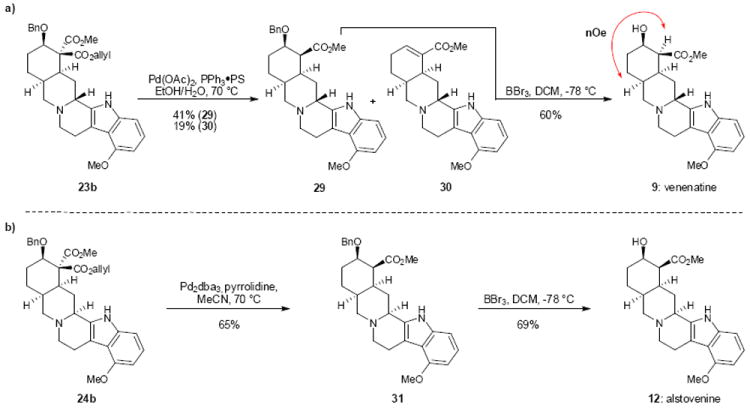
Synthesis of venenatine and alstovenine. Pd catalyzed deallylation/decarboxylation allows for allyl ester removal in 23b and 24b under mild conditions, minimizing β-hydroxy elimination. Treatment of 29 and 31 with BBr3 leads to selective removal of the benzyl protecting group and access to venenatine and alstovenine respectively. PS, polymer supported; nOe, nuclear Overhauser effect; dba, dibenzylideneacetone; Bn, benzyl; DCM, dichloromethane.
