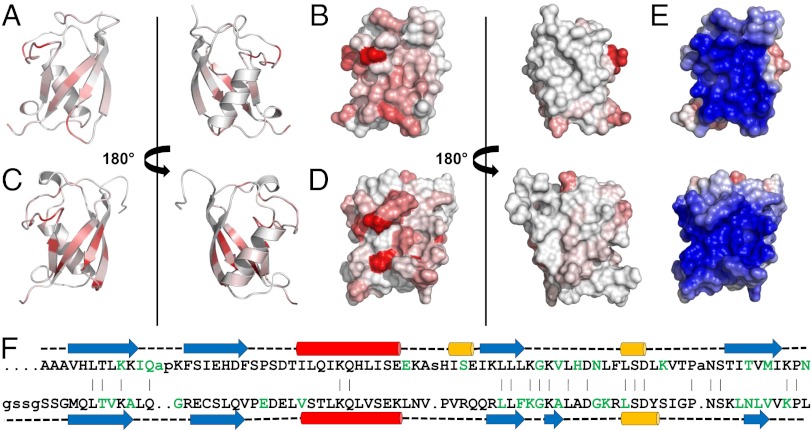
Fig. 4.
Representations of UBL domains with residues colored white to red in eight shaded increments of increasing normalized 1H/15N chemical shift perturbations observed in the presence of the equivalent SGT. Residues greater than 80% of maximum chemical shift are colored the darkest red. Between 0% and 80% are divided equally across the seven remaining shades. (A) Ribbon and (B) surface views of Get5_UBL binding interface with Sgt2_NT; (C) ribbon and (D) surface views of Ubl4a_UBL binding interface with Sgta_NT; (E) surface views of UBL domains from Get5 (Upper) and Ubl4a (Lower) colored by electrostatics; (F) structure-based sequence alignment of the UBL domains from Get5 (Upper) and Ubl4a (Lower). Red, α-helix; blue, β-strand; yellow, η-helix; green, residues perturbed upon binding to equivalent SGT protein.