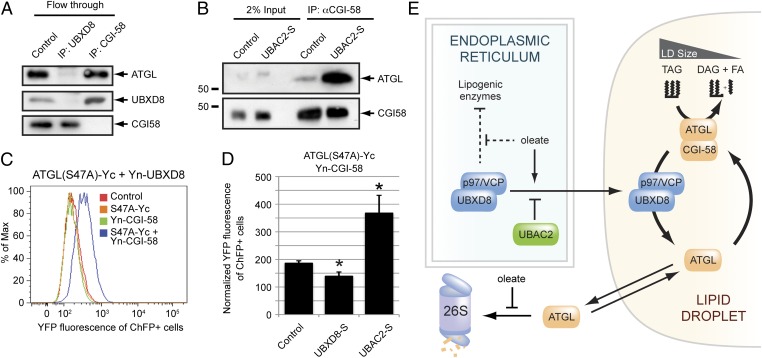
Fig. 6.
UBXD8 regulates the association of ATGL with its activator CGI-58. (A) Endogenous ATGL is preferentially associated with UBXD8 over CGI-58. Immunoblot analysis of endogenous ATGL, UBXD8, or CGI-58 in LD-enriched fractions from oleate-treated HEK293 cells immunodepleted with the indicated antibodies. (B) Depletion of endogenous UBXD8 from LDs promotes the interaction of CGI-58 and ATGL. Immunoblot analysis of endogenous CGI-58 complexes from LD-enriched fractions isolated from oleate-treated control or UBAC2-S–expressing HEK293 cells. (C) Assessment of the ATGL–CGI-58 interaction by bimolecular fluorescence complementation. HEK293 cells transiently transfected with the indicated C-terminal (Yc) or N-terminal (Yn) “split YFP” constructs were treated with 200 µM oleate for 24 h and analyzed by flow cytometry. (D) LD-associated UBXD8 modulates the interaction between ATGL and CGI-58. Quantification of flow cytometry data of ATGL(S47A)-Yc and Yn-CGI-58 bimolecular fluorescence complementation in oleate-treated HEK293 cells expressing the indicated constructs. Normalized YFP fluorescence levels (from four independent experiments) are presented as mean ± SEM. An asterisk indicates a significant difference (P < 0.05, t test) from the control cells. (E) Model illustrating the role of UBXD8 and p97/VCP as regulators of cellular fat storage. The UBXD8–UBAC2 interaction establishes a dominant tethering mechanism that restricts a pool of UBXD8 from trafficking to LDs. In the ER, UBXD8 mediates the oleate-regulated inhibition of lipogenic proteins. Oleate treatment induces UBXD8 trafficking to LDs and stabilizes ATGL by inhibiting its constitutive proteasomal degradation. On LDs, UBXD8 binds ATGL and, through its recruitment of p97/VCP, functions as a noncompetitive inhibitor that mediates dissociation of the activated ATGL–CGI-58 complex.