Abstract
A case of an asymptomatic 32-year-old male with a complex congenital pulmonary vein varix is reported herein. Chest X-ray incidentally revealed a tubular opacity passing from the periphery of the left lingula to the mediastinum. ECG gated multidetector computed tomography showed the opacity to be a vessel emptying into the left atrium via the left superior pulmonary vein. In addition, a second vascular structure was noted within the posterior mediastinum that was emptying into the same pulmonary vein. These findings were also confirmed by magnetic resonance imaging, 4D magnetic resonance angiography and invasive arterial angiography. Based on multimodality imaging findings the diagnosis of complex congenital pulmonary venous varix with posterior mediastinal extension was established.
Keywords: pulmonary vein varix, varix, mediastinum, venous structure, mediastinal tissue
CASE REPORT
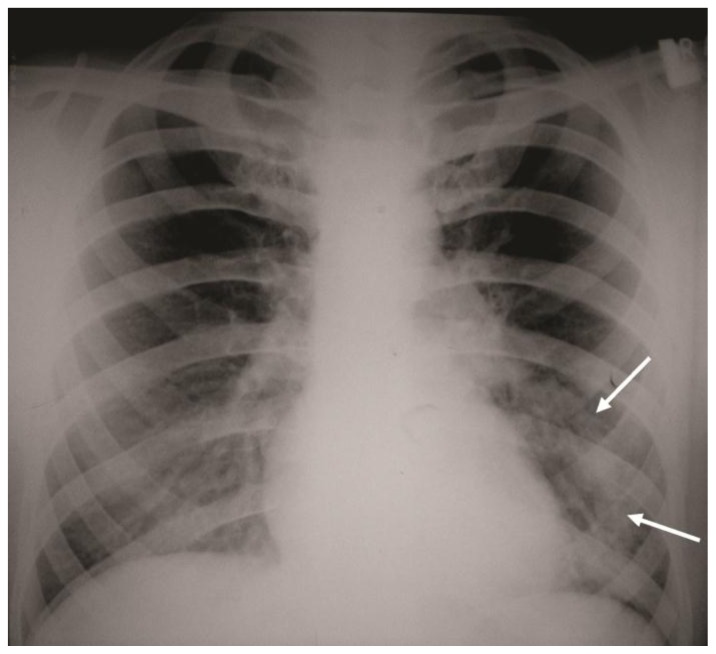
A 32-year-old male underwent a postero-anterior (PA) chest radiograph as a part of routine preoperative evaluation before appendectomy. On the PA chest radiograph an incidental finding of a tubular opacity was noted crossing from the periphery of the left middle lung towards the mediastinum (Figure 1). The patient denied any symptoms related to the cardiopulmonary system and did not have any prior history of cardiovascular disease. The physical examination of the cardiopulmonary system was normal as well. A single slice, contrast enhanced computer tomography (CT) was performed showing that the opacity represents a vessel originating in the periphery of inferior lingular segment and emptying into the left atrium via the left superior pulmonary vein.
Figure 1.
32-year-old male with complex congenital pulmonary vein varix. Chest X-Ray, PA view shows an abnormal tubular opacity in the left mid and lower lung zones. (arrows). The opacity passes from the level of the left hilum distally towards the mid portion of the left diaphragm.
Based on initial CT findings, the diagnoses of Scimitar syndrome and anomalous pulmonary venous return were excluded. In order to reach a definite diagnosis pulmonary arterial angiography using ECG gated multidetector computer tomography (MDCT) with a 64-slice scanner was employed. The initial pulmonary angiographic phase revealed no evidence of dilated systemic/bronchial arteries or pulmonary arteriovenous shunting, thus excluding bronchial artery-pulmonary vein fistula and pulmonary arteriovenous malformation (AVM). ECG gated MDCT showed a dilated left pulmonary venous structure originating from the inferior lingular segment and draining into the left atrium via the left superior pulmonary vein (Figure 2,3,4). In addition there was a second enhancing tubular vascular structure within the posterior mediastinum emptying into the very proximal portion of the left superior pulmonary vein. This structure had a tortuous course and it was formed from few smaller vessels originating from a surrounding area of soft tissue density. Based on these findings the differential diagnosis was narrowed to a complex pulmonary venous varix-acquired versus congenital with an extension into the posterior mediastinum. For further confirmation and investigation of the posterior mediastinal findings MRI of the heart and vessels was performed on 1,5T MRI machine. The soft tissue within the posterior mediastinum, surrounding the tortuous posterior mediastinal vessels was hyperintense on T1w images and showed no significant enhancement following administration of gadolinium (Figure 5). This is most likely consistent with fibrotic tissue. MRI of the heart showed normal morphology as well as kinetics of the heart and no evidence of valvular disease. Ventricular function was within normal limits. Velocity-encoded cine (VEC) MRI was performed through aorta and main pulmonary artery showing no difference between amount of flow through them (Qp=Qs), which proved no evidence of shunting. Also there was no evidence of pericardial disease or delayed myocardial enhancement. 4D MR angiography (MRAG) showed that the dilated left pulmonary venous structure originating in lingula filled at the same time as the rest of the normal pulmonary veins. On the delayed MRAG images this left pulmonary venous structure remained more hyperintense in comparison to other normal pulmonary veins, consistent with delayed emptying. (Figure 6). These MRI and MRAG findings reaffirmed the diagnosis of a complex PVV. Since there was no associated pathology of the left heart which could cause increase in left pulmonary venous pressure the nature of the PVV was considered to be congenital.
Figure 2.
32-year-old male with complex congenital pulmonary vein varix performed on 64 slice Dual Energy CT scanner (outside hospital) in arterial phase: MIP reformat in coronal projection using mediastinal vascular window shows a dilated left pulmonary venous structure (solid white arrow) originating from the inferior lingular segment and draining into the left atrium via the left superior pulmonary vein (dotted white arrow).
Protocol: 100mAs, 100kV, 3mm slice thickness, 80ml Ultravist 370
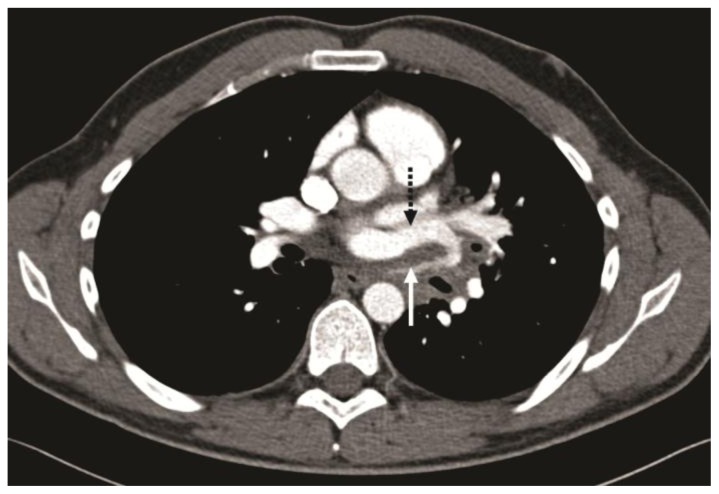
Figure 3.
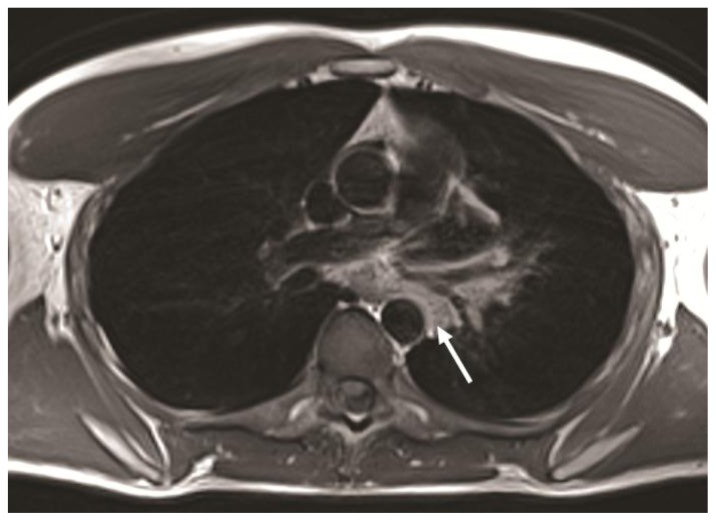
32-year-old male with complex congenital pulmonary vein varix. ECG gated MDCT performed on 64 slice CT scanner: Contrast enhanced axial image at the level of pulmonary artery origin obtained during pulmonary arterial phase using a mediastinal vascular window shows tortuous vessel in the posterior mediastinum passing between left superior pulmonary vein and descending aorta (solid white arrow) and draining into the left atrium via the left superior pulmonary vein (dotted black arrow).
Protocol: 40mAs, 120kV, 0.7mm slice thickness, 80ml Ultravist 370
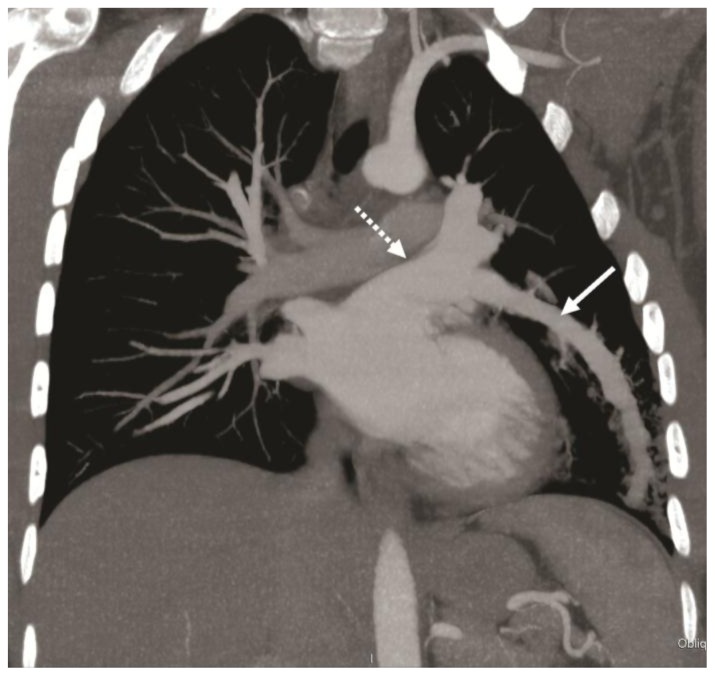
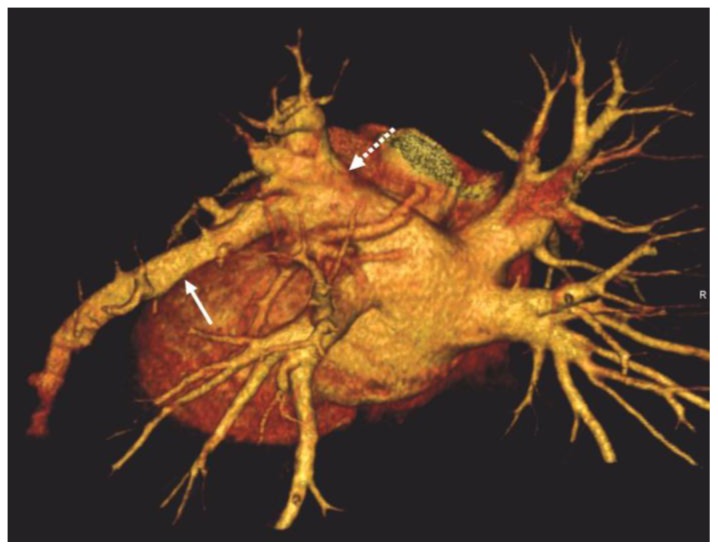
Figure 4.
32-year-old male with a diagnosis of complex congenital pulmonary vein varix. ECG gated MDCT performed on 64 slice CT scanner: LPO (left posterior oblique) view of a volume rendered reformat of the pulmonary veins and left atrium in posterior projection shows a dilated left pulmonary venous structure (solid white arrow) originating from the inferior lingular segment and draining into the left atrium via the left superior pulmonary vein (dotted white arrow).
Protocol: 40mAs, 120kV, 0.7mm slice thickness, 80ml Ultravist 370
Figure 5.
32-year-old male with a diagnosis of complex congenital pulmonary vein varix. MR of the mediastinum acquired on 1.5 Tesla MR scanner. Non-contrast T1 weighted turbo spin echo (TSE) dark blood (DB) axial image of mediastinum at the level of right pulmonary artery showing hyperintense tissue within the mediastinum. The tissue is localized adjacent to the anterior and left side of the aorta and it is surrounding the visualized portion of the left superior pulmonary vein.
Protocol: T1 TSE DB, TE 28, TR 1073, single slice
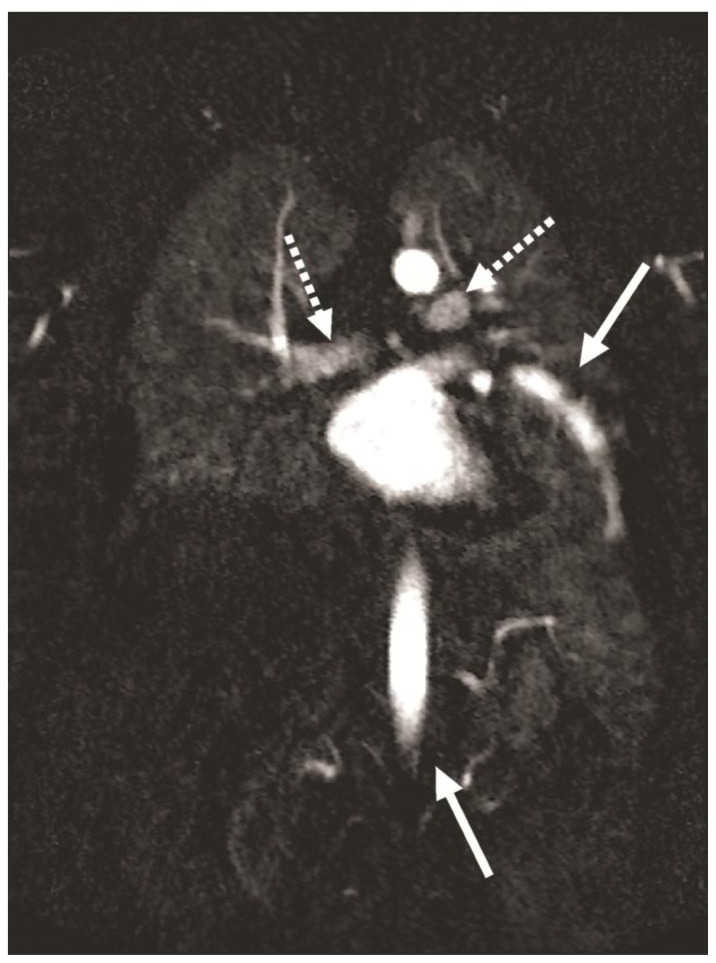
Figure 6.
32-year-old male with complex congenital pulmonary vein varix. Maximum intensity projection (MIP) reconstruction of Time-resolved Angiography With Stochastic Trajectories (TWIST) 4D MR angiography (MRAG) on 1.5 Tesla MR scanner 68.6 sec after contrast injection in coronal view demonstrating lesser enhancement of pulmonary vein bilaterally (dotted arrows) compared to persistent enhancement of dilated vein (arrow) in aortic phase (second arrow), which confirms its delayed emptying.
Protocol: TE 1, TR 2.5, slice thickness 2.5 mm, Contrast material - Gadovist- 10ml
A follow-up 4D MRAG on a 3T MRI machine performed 6 month later showed similar findings, coupled with better delineation of more peripheral tortuous vessels emptying into distal portion of the dilated PVV. (Figure 7). Since the origin of previously noted tortuous vessels, systemic arterial versus pulmonary venous, could not be determined with full certainty based on MDCT and MRI findings, IAG, was performed. IAG ruled out communication between the presumed left PVV and bronchial arteries or any other systemic aortic branches. (Figure 8). Therefore the diagnosis of PVV of congenital origin was finally confirmed. Cumulative effective dose of used imaging methods was 26.8 mSv (PA chest radiograph, CT from outside hospital, ECG gated MDCT and IAG of the aorta and pulmonary artery with effective dose of 0.2, 13.3, 7.1 and 6.2 mSv respectively).
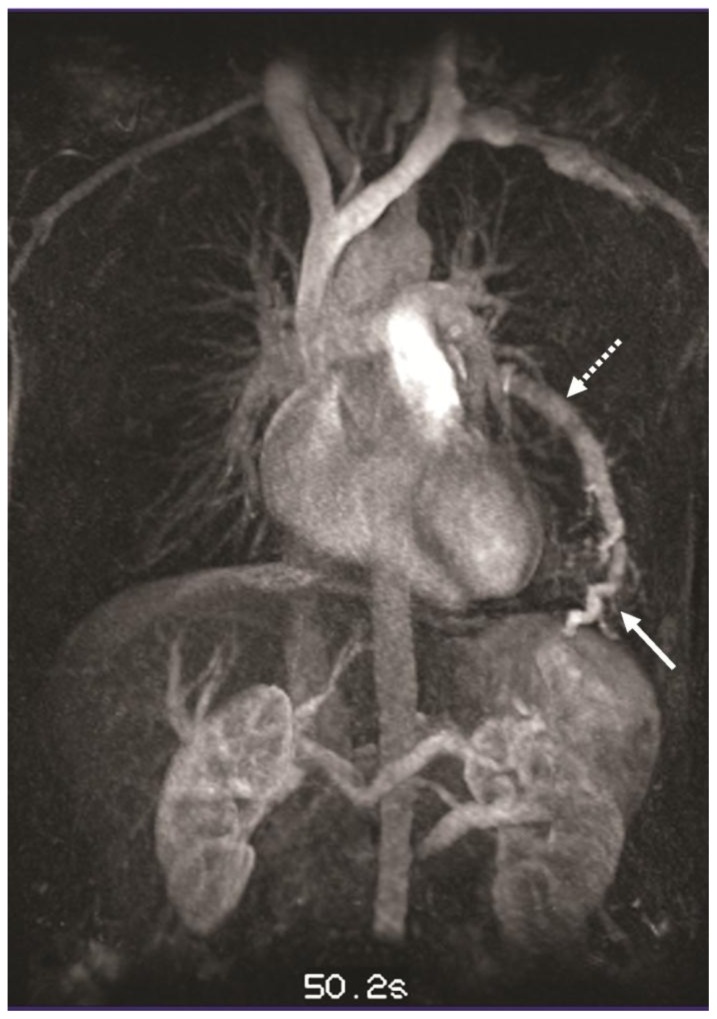
Figure 7.
32-year-old male with complex congenital pulmonary vein varix. Follow up MIP reconstruction of TWIST 4D MRAG performed on 3T MR machine at 50.2 sec after contrast injection in coronal view demonstrates simultaneous enhancement of the pulmonary veins, the dilated left pulmonary vein varix (dotted white arrow) and a peripheral tortuous pulmonary venous structure emptying into the left pulmonary vein varix (white solid arrow).
Protocol: TE 0.95, TR 2.57, slice thickness 3mm, Contrast material - Gadovist- 10ml
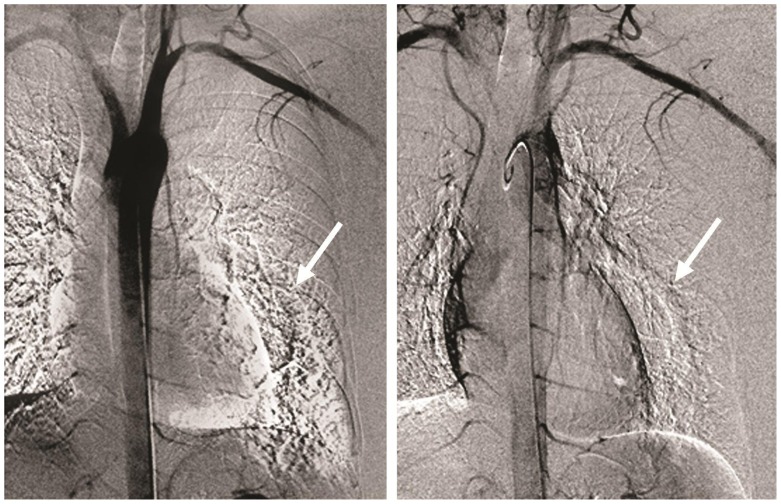
Figure 8.
32-year-old male with a diagnosis of complex congenital pulmonary vein varix. Invasive aortic angiography (IAG) in aortic phase showed neither evidence of dilated aortic branches nor communication and filling of PVV with contrast material in this phase. Arrows are pointing to the area of dilated pulmonary vein.
Digital subtraction angiography, Contrast: Optiray- 100ml
DISCUSSION
Congenital anomalies of the pulmonary veins, which cause their dilatation include Scimitar syndrome, partial anomalous pulmonary venous return (PAPVR), pseudoscimitar syndrome and pulmonary vein varix. In Scimitar syndrome dilated pulmonary vein drains into the inferior vena cava or rarely on left side to the azygos vein. It is commonly associated with hypogenetic lung and dextrocardia. In case of PAPVR dilated pulmonary vein drains to the inferior vena cava or to the right atrium. Scimitar syndrome and PAPVR are cause of left to right shunt. Pseudoscimitar syndrome is characterized by dilated pulmonary vein which drains directly into left atrium. Acquired dilation of the pulmonary vein is due to increased pulmonary venous pressure from increased pressure in the left atrium (e.g.mitral valve insufficiency), or due to a pulmonary arteriovenous malformation (PAVM), or less common communication between bronchial artery and pulmonary vein.
The latest embryological research shows [1] that the pulmonary veins arise as solitary vessels in the mesenchyma of the mediastinum and canalize the blood of the pulmonary vein plexus into the heart. The individual pulmonary veins that have been engendered become incorporated into the left atrium and finally 4 independent pulmonary veins empty into the heart.
Aneurysmal dilatation of the pulmonary vein, otherwise known as PVV [2], is a rare entity. Only 71 such cases have been reported. Congenital PVV probably results [3] from dilatation of persistent embryological venous drainage channels. Histological examination of a PVV suggests no intrinsic defect in the structure of the wall. Acquired PVV [4] are due to valvular disease or in patients with end-stage liver disease or portal hypertension. Pulmonary varices may be classified into three types, namely: saccular type (localized, ovular form), tortuous type (extensive and irregular) and confluent type (localized at the confluence of the pulmonary veins). Most of the varices seen in patients with valvular disease have been of the confluent type (62 per cent), however tortuous type varices have also been seen in some cases (19 per cent). Pulmonary venous hypertension may be one of the major causes of confluent type pulmonary varices as regression of pulmonary varices after mitral valve replacement has been reported. None of the saccular type cases, however, were accompanied by valvular disease. This indicates that local factors [3] may also be an important cause of saccular type varices.
Pulmonary varices usually remain stable in the absence of pulmonary venous hypertension. Acute increase in varix size is indicative of elevation of left atrial pressure. Complications of pulmonary varices include rupture, thromboembolism and hemoptysis. Most varices secondary to pulmonary venous hypertension usually regress with correction of the pulmonary venous hypertension, e.g. mitral valve replacement in patients with mitral valve disease. In cases of hemoptysis [3] due to non-cardiac causes, pulmonary lobe resection may be necessary. Nonetheless in most cases treatment of pulmonary varices is usually unnecessary once the correct diagnosis is established. Radiological monitoring [5] is essential. PVV that increase in size or show a risk of complications [3] are candidates for surgical intervention.
Pulmonary varix [6] has been noted in all age groups. Its frequency is equal in males and females. Of a total of 71 cases, the lesion was situated [6] in the right lower lobe in 43 patients (60%), in the left upper lobe in 12 (17%), in the right upper lobe in 6 (8%), in the right middle lobe in 3 (4%) and in the left lower lobe in 3 patients (4%)
On invasive pulmonary angiography the diagnostic criteria of PVV include: (1) normal pulmonary arterial tree without dilatation; (2) the pulmonary vein draining the varix and the varix itself fill at the same rate as the normal pulmonary veins; (3) the varix drains into the left atrium, so there is no evidence of shunt between systemic and pulmonary circulations; (4) there is delayed emptying of the varix compared to other normal pulmonary veins; (5) the tortuous course of a pulmonary varix affects only its proximal portion. Diagnosis of a PVV can be established also by ECG gated MDCT and MRI, using their superior multiplanar capability, when the aforementioned diagnostic criteria of invasive pulmonary angiography are fulfilled. MRAG can further demonstrate [7] the dynamics of blood flow between the PVV, the pulmonary vein draining the PVV and the left atrium.
In our case both ECG gated MDCT and MRI confirmed the presence of a left PVV draining into the left atrium via the superior left pulmonary vein. Furthermore they documented that the left superior pulmonary vein draining the PVV and the PVV itself filed at the same rate as the normal pulmonary veins with delayed emptying of the PVV compared to other normal pulmonary veins.
This case is of particular interest, since both ECG gated MDCT and MRI confirmed presence of vessels in the posterior mediastinum that drained into the left atrium via the left superior pulmonary vein. There was also a tortuous vessel draining into the distal portion of the dilated vein. This vessel could have been systemic arterial in nature and the possibility of a bronchial arterial-pulmonary vein fistula had to be excluded via IAG. Since there was no communication between the aorta or its branches and the dilated left pulmonary venous structure the diagnosis of a PVV was finally confirmed. Since MRI revealed no evidence of mitral valve disease or other causes of pulmonary vascular hypertension, we excluded the possibility of an acquired PVV and determined it to be congenital in nature.
Considering the normal embryological development of the pulmonary veins, the previously noted tortuous vessels in the posterior mediastinum that drained into the left atrium via the left superior pulmonary vein, most likely represent another developmental anomaly of the pulmonary vein. We postulate that this vein developed from the mesenchyma of the posterior mediastinum, became incorporated into the left atrium, but failed to unite with the angioblastic plexus of the developing left lower lung.
TEACHING POINT
Pulmonary vein varix (PVV) of congenital origin is an anomalous dilatation of the pulmonary vein with no evidence of increased venous pressure, which fills at the same rate as the normal pulmonary veins and drains into the left atrium with a delay compared to other normal pulmonary veins. It is a very rare entity and the correct diagnosis is crucial, since congenital PVV, unlike other pathologies responsible for dilatation of pulmonary vein, does not require any treatment and intention to do so can potentially harm the patient.
Table 1.
Summary table of pulmonary vein varix
| Etiology | Congenital |
| Incidence | Only 71 cases were published |
| Gender ratio | Equal in males and females |
| Age prediction | Noted in all age groups |
| Risk factors | Not known |
| Treatment | Not required unless there are complications |
| Prognosis | Usually remains stable |
| Findings on imaging | Dilated pulmonary vein is visualized as a tubular opacity on chest X-ray, on CTAG, MRAG or IAG drains into left atrium, fills at the same rate as other pulmonary veins, and shows delayed emptying compare to other pulmonary veins |
| Side prediction | right lower lobe 60% left upper lobe in 17% right upper lobe in 8% right middle lobe in 4% left lower lobe in 4% |
| Possible complications | rupture, thromboembolism and hemoptysis |
Table 2.
Differential table of pulmonary vein varix
| Differential diagnosis | X-ray | CT | MR(MRAG) | IAG |
|---|---|---|---|---|
| Scimitar syndrome | tubular opacity crossing from the periphery of lung towards the mediastinum (usually on the right side) Hypogenetic lung, dextrocardia | dilated pulmonary vein draining to inferior vena cava or rarely on left to azygos vein hypogenetic lung, dextrocardia | dilated pulmonary vein draining to inferior vena cava or rarely on left to azygos vein hypogenetic lung, dextrocardia, left to right shunt | dilated pulmonary vein draining to inferior vena cava or rarely on left to azygos vein hypogenetic lung, dextrocardia, left to right shunt |
| Pseudoscimitar syndrome | tubular opacity crossing from the periphery of lung towards the mediastinum (usually on the right side) | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium |
| Partial anomalous pulmonary venous return (PAPVR) | tubular opacity crossing from the periphery of lung towards the mediastinum | dilated pulmonary vein draining to inferior vena cava or other way to right atrium | dilated pulmonary vein draining to inferior vena cava or other way to right atrium, left to right shunt | dilated pulmonary vein draining to inferior vena cava or other way to right atrium, left to right shunt |
| Pulmonary vein varix (PVV) Acquired | tubular opacity crossing from the periphery of lung towards the mediastinum | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium |
| 1 sign of below mentioned | ||||
| arteriovenous malformation | arteriovenous malformation | arteriovenous malformation | ||
| bronchial artery- pulmonary vein fistula | bronchial artery- pulmonary vein fistula | bronchial artery- pulmonary vein fistula | ||
| valvular disease - mitral valve | increase pressure in the left atrium | |||
| Pulmonary vein varix (PVV) Acquired | tubular opacity crossing from the periphery of lung towards the mediastinum | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium | dilated pulmonary vein draining into left atrium |
| dilated vein fills at the same rate as other pulmonary veins | dilated vein fills at the same rate as other pulmonary veins | |||
| delayed emptying of the dilated vein compare to other pulmonary veins | delayed emptying of the dilated vein compare to other pulmonary veins | |||
| no proven reason for the pulmonary vein dilatation | ||||
ABBREVIATIONS
- 3T
3 Tesla
- AVM
arteriovenous malformation
- CT
computer tomography
- IAG
invasive angiography
- MIP
maximum intensity projection
- MDCT
multidetector computer tomography
- MRAG
MR angiography
- MRI
magnetic resonance imaging
- PA
postero-anterior
- PAPVR
partial anomalous pulmonary venous return
- PAVM
pulmonary arteriovenous malformation
- TWIST
Time-resolved Angiography With Stochastic Trajectories
- VEC
Velocity-encoded cine
REFERENCES
- 1.On-line course in embryology for medicine students developed by the universities of Fribourg, Lausanne and Bern (Switzerland)with the support of the Swiss Virtual Campus, Development of the veins. [Accessed August 25, 2011]. Available at: http://www.embryology.ch/anglais/pcardio/venen04.html.
- 2.Tadashi Uyama, Yasumasa Monden, Kunihiko Harada. Pulmonary varices: A case report and review of the literature. Surgery Today. 1998;18:359–362. doi: 10.1007/BF02471456. [DOI] [PubMed] [Google Scholar]
- 3.Jun Shiraishi, Tetsuya Tatsumi, Masaki Kimata, et al. Echocardiographic Dagnosis of Pulmonary Vein Varix. Circ J[serial online] 2003;67:796–798. doi: 10.1253/circj.67.796. [DOI] [PubMed] [Google Scholar]
- 4.Man KM, Keeffe EB, Brown CR, et al. Pulmonary varices presenting as a solitary lung mass in a patient with end-stage liver disease. [Accessed September 25, 2011];Chest [serial online] 1994 106:294–296. doi: 10.1378/chest.106.1.294. Available at: http://chestjournal.chestpubs.org/content/106/1/294.long. [DOI] [PubMed] [Google Scholar]
- 5.Sellarés J, Santos S, Ballester E, et al. CASE REPORTS Pulmonary Varix Inside a Bulla. Arch Bronconeumol. [serial online] 2006;42(1):39–41. doi: 10.1016/s1579-2129(06)60112-2. [DOI] [PubMed] [Google Scholar]
- 6.Kumazoe Hiroyuki, Komori Masashi, Ochiai Reiji, Egashira Ryoko, Nakazono Takahiko, Kudo Sho. Pulmonary varix mimicking arteriovenous malformation. Clinical Imaging. 2008;32:61–64. doi: 10.1016/j.clinimag.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Kaur M. Thoracic Case Report 2. [New York University, School of Medicine, Department of Radiology web site]. December 29, 2003 and January 22, 2004. [Accessed September 26, 2011]. Available at: http://www.med.nyu.edu/mri/thoracic/case02.html.