Abstract
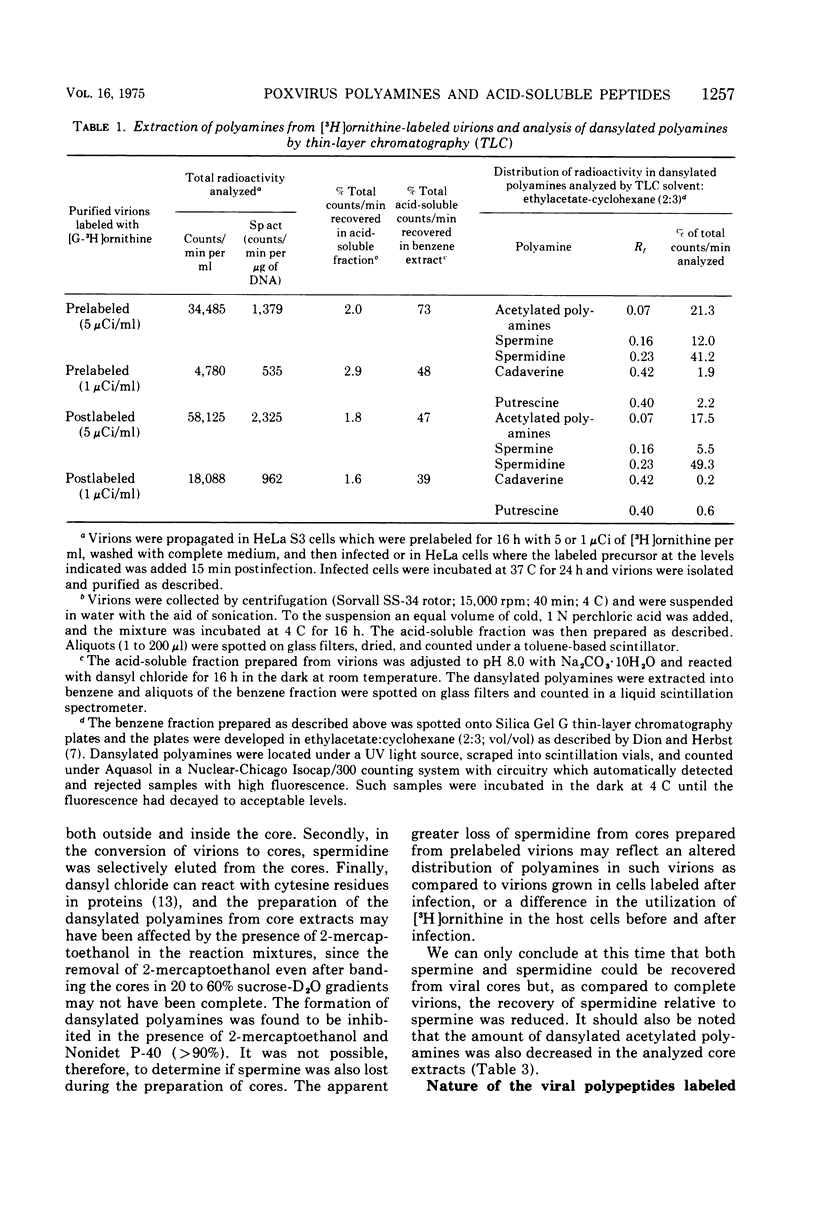
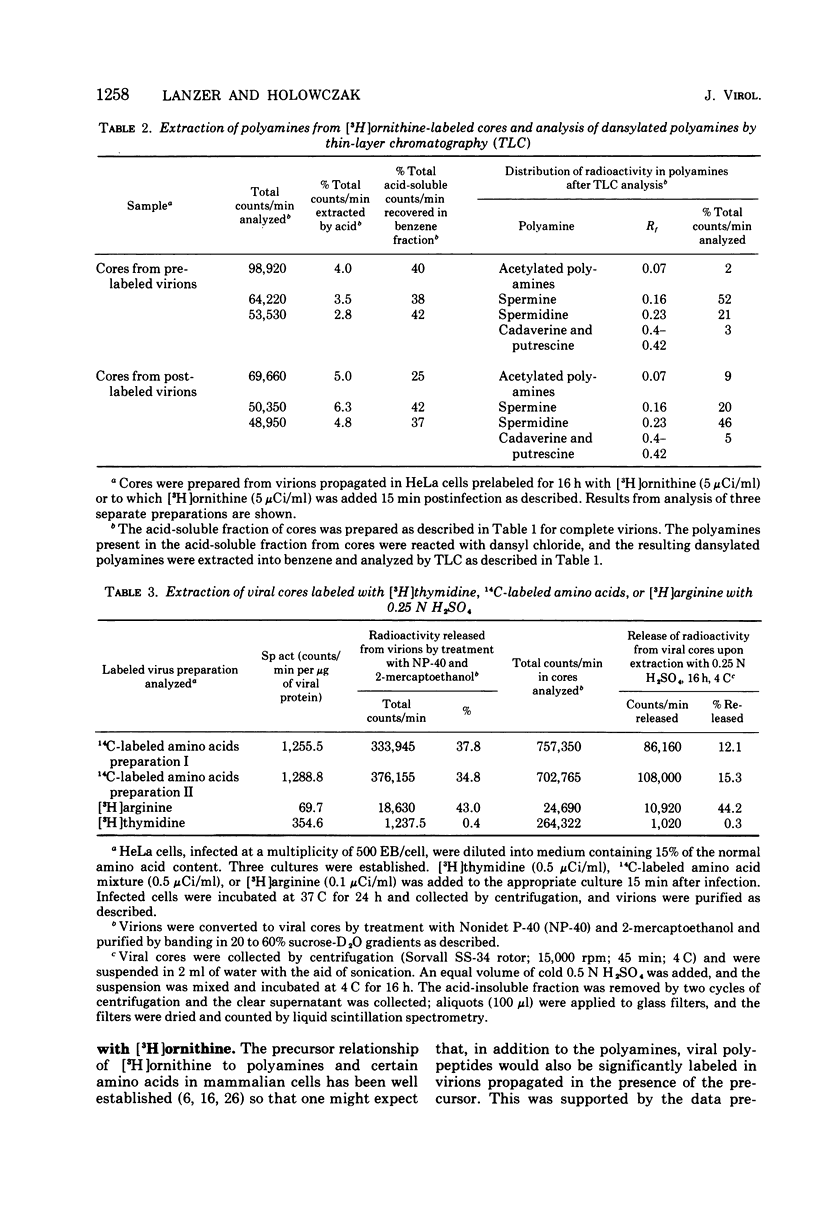
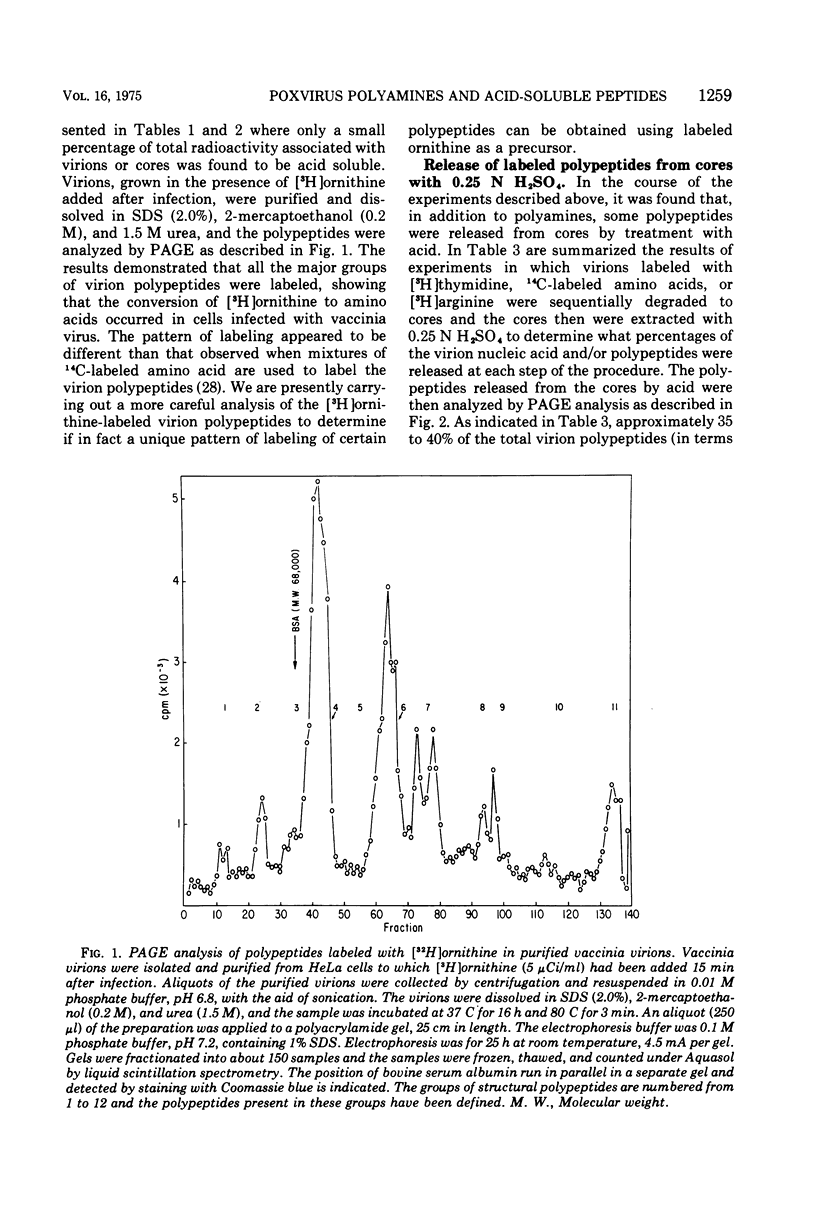
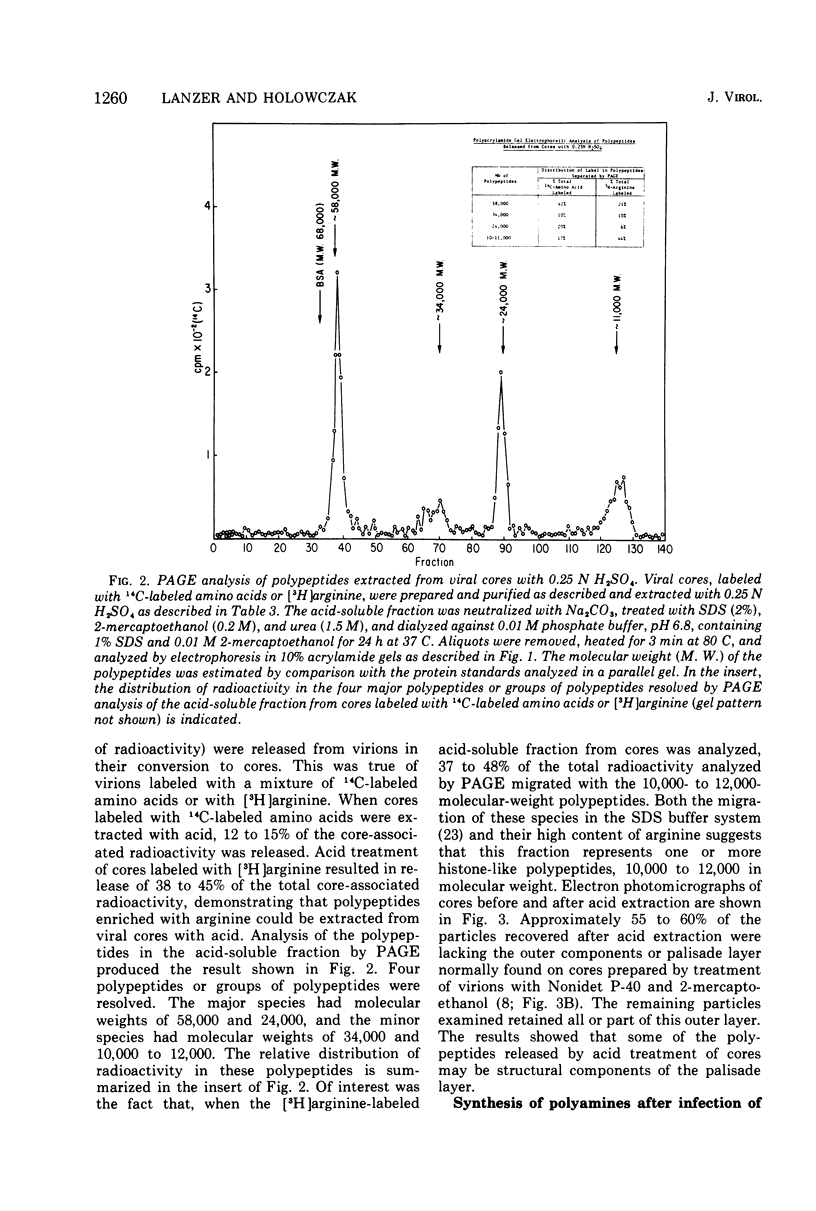
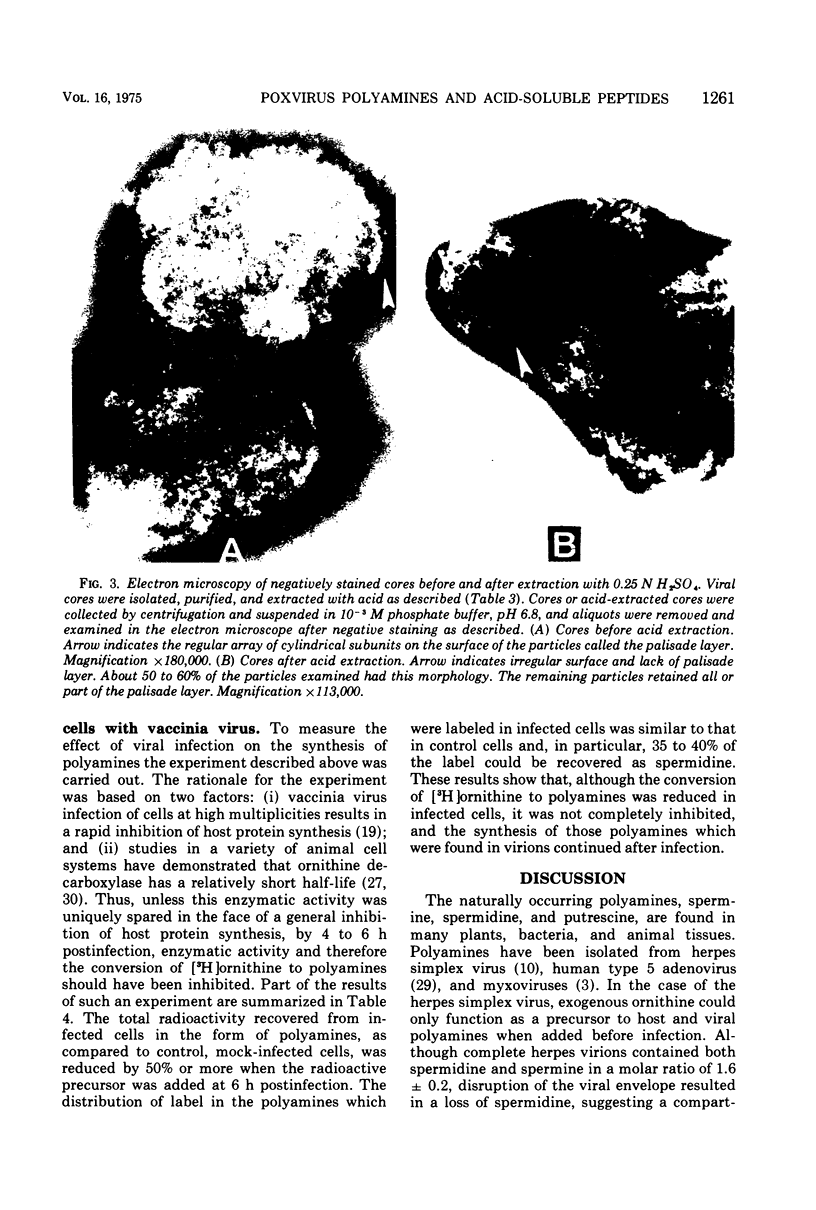
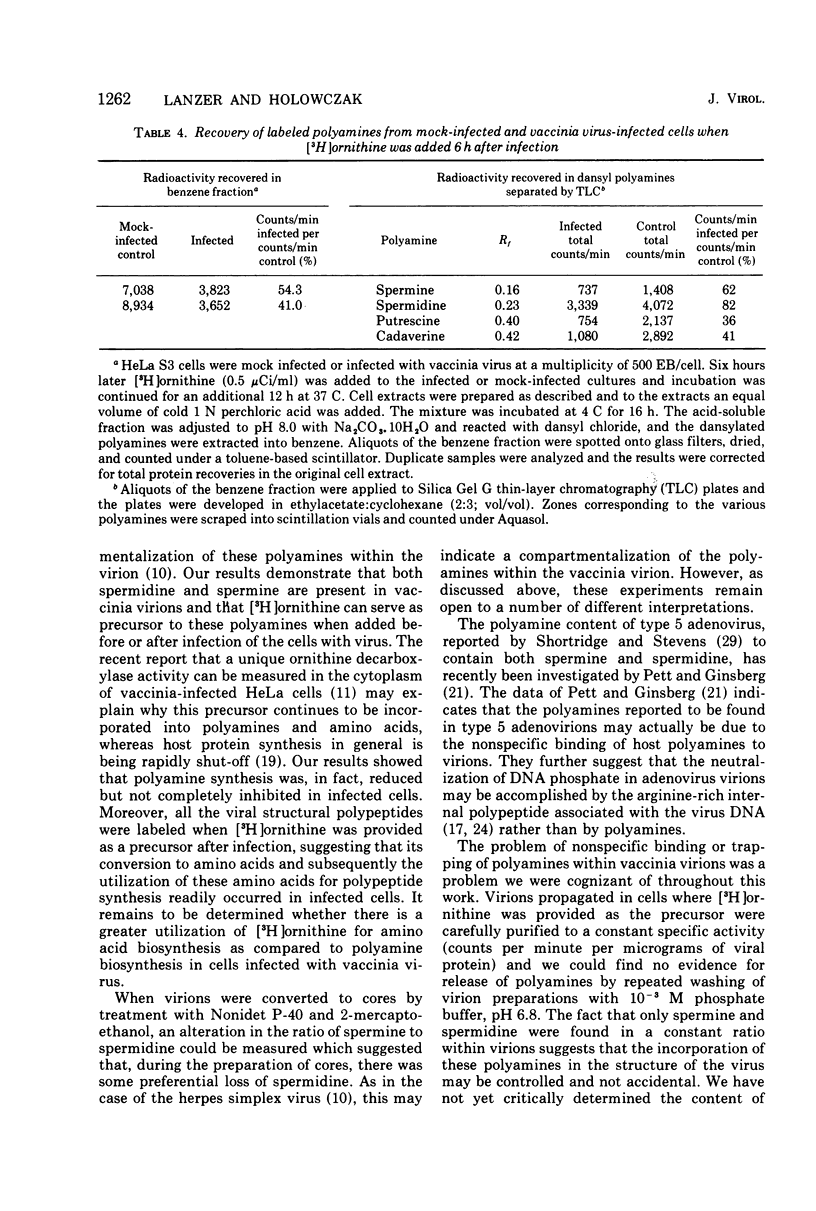
Vaccinia virions propagated in the presence of [3H]ornithine were found to contain two labeled polyamines, spermine and spermidine. In complete virions the ratio of radioactively labeled spermine to spermidine was about 1:10, whereas in viral cores the ratio was 2:5. This suggests that some spermidine was preferentially lost during the conversion of virions to cores or that spermidine was present in the virions both inside and outside the core structure. Addition of [3H]ornithine to vaccinia virus-infected cells as late as 6 h postinfection demonstrated that, although the conversion of this precursor to polyamines was reduced by 50% or more as compared to mock-infected cells, complete inhibition of polyamine synthesis did not occur. Two percent or less of the total radioactivity associated with virions grown in the presence of [3H]ornithine was found to be acid soluble. Polyacrylamide gel electrophoretic analysis showed that all the structural polypeptides were labeled when virions were propagated in the presence of [3H]ornithine. When cores labeled with a mixture of 14C-labeled amino acids were extracted with 0.25 N H2SO4, 12 to 15% of the labeled core polypeptides were released and could be precipitated with acetone. About 40% of [3H]arginine-labeled polypeptides associated with cores were extracted with acid. Four polypeptides or groups of polypeptides were resolved after polyacrylamide gel electrophoresis analysis of the acid-soluble fraction of cores with molecular weights of about 58,000, 34,000, 24,000 and 10,000 to 12,000. About 40% of the [3H]arginine radioactivity extracted from cores coelectrophoresed with the 10,000 to 12,000-molecular weight polypeptide, indicating that this may represent an arginine-rich, histone-like structural polypeptide of the virion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U., Don S., Wiener H. Occurrence of polyamines in myxoviruses. J Gen Virol. 1974 Mar;22(3):451–454. doi: 10.1099/0022-1317-22-3-451. [DOI] [PubMed] [Google Scholar]
- Beer S. V., Kosuge T. Spermidine and spermine--polyamine components of turnip yellow mosaic virus. Virology. 1970 Apr;40(4):930–938. doi: 10.1016/0042-6822(70)90139-x. [DOI] [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2818–2821. doi: 10.1073/pnas.68.11.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J., Williamson J. D. Ornithine decarboxylase activity in uninfected and vaccinia virus-infected HeLa cells. Biochem Biophys Res Commun. 1975 Mar 3;63(1):308–312. doi: 10.1016/s0006-291x(75)80044-1. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. W., MARKHAM R. Nature of the polyamine in plant viruses. Virology. 1962 Jun;17:276–281. doi: 10.1016/0042-6822(62)90117-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Ginsberg H. S. Polyamines in type 5 adenovirus-infected cells and virions. J Virol. 1975 May;15(5):1289–1292. doi: 10.1128/jvi.15.5.1289-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Katz J. R., Dales S. Biogenesis of poxviruses: synthesis and phosphorylation of a basic protein associated with the DNA. Virology. 1975 Apr;64(2):531–543. doi: 10.1016/0042-6822(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Roening G., Holowczak J. A. Evidence for the presence of RNA in the purified virions of vaccinia virus. J Virol. 1974 Sep;14(3):704–708. doi: 10.1128/jvi.14.3.704-708.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Shortridge K. F., Stevens L. Spermine and spermidine--polyamine components of human type 5 adenovirions. Microbios. 1973;7(25):61–68. [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]