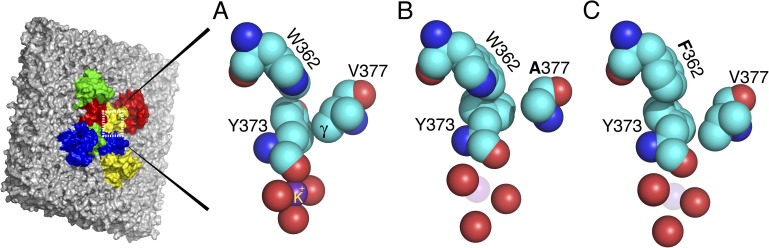
Figure 3.
An expanded view of the outer mouth of the selectivity filter of the Kv1.2-2.1 chimera and two simulated mutations. The four outermost Y373 carbonyl oxygens outline the pore, but only one tyrosine is shown in full. (A) The conducting conformation of the filter mouth, taken from the Kv1.2-2.1 chimera. Y373 (Y445) has close contacts with W362 (W434) and V377 (T449). (B) A simulated mutation of the Kv1.2-2.1 chimera that replaces V377 (T449) with V377A (T449A), allowing Y373 (Y445) to rotate and dilate the filter mouth. The mutation in effect deletes the two γ carbons of V377 (T449), removing a contact that locks Y373 (Y445) in the conducting, nondilated position. (C) Simulated mutation W362 (W434) of the Kv1.2-2.1 chimera that replaces W362 (W434) with F362 (F434). Relative to W362 (W434), F362 (F434) is rotated clockwise in this view; it presses against the ring of Y373 (Y445), causing Y373 (Y445) to rotate counterclockwise, dilating the filter mouth. The angle of view is the same throughout the figure. The images were prepared using MacPyMol (version 0.99).