Abstract
Protein kinase C (PKC) has been implicated in the regulation of smooth muscle cell (SMC) contraction and may contribute to airway hyperresponsiveness. Here, we combined optical and biochemical analyses of mouse lung slices to determine the effects of PKC activation on Ca2+ signaling, Ca2+ sensitivity, protein phosphorylation, and contraction in SMCs of small intrapulmonary airways. We found that 10 µM phorbol-12-myristate-13-acetate or 1 µM phorbol 12,13-dibutyrate induced repetitive, unsynchronized, and transient contractions of the SMCs lining the airway lumen. These contractions were associated with low frequency Ca2+ oscillations in airway SMCs that resulted from Ca2+ influx through L-type voltage-gated Ca2+ channels and the subsequent release of Ca2+ from intracellular stores through ryanodine receptors. Phorbol ester stimulation of lung slices in which SMC intracellular Ca2+ concentration ([Ca2+]i) was “clamped” at a high concentration induced strong airway contraction, indicating that PKC mediated sensitization of the contractile response to [Ca2+]i. This Ca2+ sensitization was accompanied by phosphorylation of both the PKC-potentiated PP1 inhibitory protein of 17 kD (CPI-17) and the regulatory myosin light chain. Thrombin, like the phorbol esters, induced a strong Ca2+ sensitization that was inhibited by the PKC inhibitor GF-109203X and also potentiated airway contraction to membrane depolarization with KCl. In conclusion, we suggest that PKC activation in small airways leads to both the generation of Ca2+ oscillations and strong Ca2+ sensitization; agents associated with airway inflammation, such as thrombin, may activate this pathway to sensitize airway smooth muscle to agonists that cause membrane depolarization and Ca2+ entry and induce airway hyperresponsiveness.
INTRODUCTION
The small intrapulmonary airways are the major site of airflow limitation in both asthma and chronic obstructive pulmonary disease (Burgel, 2011; McDonough et al., 2011). However, the small size and peripheral location of these small distal airways, together with their mechanical interactions with the lung parenchyma, have made it challenging to determine the cellular mechanisms that regulate their diameter in normal and diseased lungs. Recent studies using customized imaging techniques to assess dynamic changes in airway diameter simultaneously with changes in smooth muscle cell (SMC) intracellular Ca2+ concentration ([Ca2+]i) in lung slices, however, have begun to provide insight into the processes that regulate small airway contraction (Sanderson, 2011).
Stimuli that increase (contractile stimuli) or decrease (relaxing stimuli) airway resistance regulate airway SMC contraction by modulating both [Ca2+]i and the contractile response of the SMC to a giving [Ca2+]i (Ca2+ sensitivity). Changes in smooth muscle Ca2+ sensitivity are mainly caused by Ca2+-independent regulation of the myosin light chain phosphatase (MLCP) activity, resulting in alterations in phosphorylation of the regulatory myosin light chain (rMLC), thereby in SMC contraction (Somlyo and Somlyo, 2003). Contractile stimuli, including ligands of G protein–coupled receptors that stimulate signaling via Gq/11 (e.g., acetylcholine [ACh] or serotonin [5-HT]) or membrane depolarization with KCl, induce Ca2+ oscillations—repetitive transient increases in [Ca2+]i—in airway SMCs (Bergner and Sanderson, 2002; Perez and Sanderson, 2005). The strength and dynamics of airway SMC contraction correlate with the frequency of these Ca2+ oscillations (Perez and Sanderson, 2005). High frequency Ca2+ oscillations are associated with sustained and synchronized SMC contraction that result in large reductions in airway lumen, whereas low frequency Ca2+ oscillations are associated with transient and unsynchronized SMC contractions (SMC twitching) that result in small reduction in airway lumen. Contractile stimuli also activate mechanisms that increase the Ca2+ sensitivity (Bai and Sanderson, 2006b). Conversely, β2 agonists and nitric oxide induce airway relaxation by decreasing the frequency of Ca2+ oscillations, the Ca2+ sensitivity, or both (Delmotte and Sanderson, 2008; Perez-Zoghbi et al., 2010).
The mechanisms underlying the generation and maintenance of Ca2+ oscillations in small airway SMCs have been reasonably well characterized (Perez-Zoghbi et al., 2009). High frequency Ca2+ oscillations in response to stimulation with contractile agonists such as ACh or 5-HT are generated by cyclic Ca2+ release from intracellular Ca2+ stores through inositol 1,4,5 triphosphate receptors (Bai et al., 2009). Ca2+ entry through store-operated Ca2+ channels replenishes the Ca2+ stores, thereby maintaining Ca2+ oscillations and sustained airway contraction during prolonged agonist stimulation (Perez and Sanderson, 2005). In contrast, membrane depolarization with KCl stimulates Ca2+ entry through L-type voltage-gated Ca2+ channels (LVGCs), leading to Ca2+ store overload and thereby to the release of Ca2+ from intracellular stores. Localized Ca2+ release events are amplified by Ca2+-induced Ca2+ release through RyRs, resulting in Ca2+ wave propagation and the simultaneous SMC twitching that characterize the small airway KCl response (Perez and Sanderson, 2005).
The mechanisms whereby contractile agonists increase Ca2+ sensitivity (Ca2+ sensitization) in small airways, however, have not been well characterized. Experiments with lung slices in which the SMCs have been made permeable to Ca2+ indicate that the intrinsic (basal) Ca2+ sensitivity of the small airways is low and that contractile agonists increase Ca2+ sensitivity (Bai and Sanderson, 2006b; Perez-Zoghbi and Sanderson, 2007). Most agonists that stimulate airway contraction are ligands of G protein–coupled receptors that signal through Gq/11 and G12/13 and consequently activate both PKC and RhoA kinase (ROK) in SMCs, respectively (Wright et al., 2012). In the small airways, inhibitors of PKC or ROK inhibit ACh-induced Ca2+ sensitization (Bai and Sanderson, 2006b). However, the specific roles of PKC and ROK in airway contraction and the mechanism underlying Ca2+ sensitization in small airways have not been investigated.
The PKC family comprises a group of isoenzymes with serine/threonine protein kinase activity that are expressed ubiquitously in mammalian cells (Sakai et al., 2009). PKC activation by inflammatory mediators may contribute to airway hyperresponsiveness in experimental asthma (Morin et al., 2012). The coagulation factor thrombin, which is increased in the small airways and the alveolar space of asthma patients compared with those of controls (Gabazza et al., 1999; Kanazawa and Yoshikawa, 2007) and causes airway hyperresponsiveness in experimental asthma (Wagers et al., 2004), also appears to be involved in the pathogenesis of asthma (de Boer et al., 2012). Thrombin induces Ca2+ sensitization of the contraction mediated by stress fibers in retinal pigment epithelial cells by activating PKC and thereby promoting phosphorylation of PKC-potentiated PP1 inhibitory protein of 17 kD (CPI-17) and rMLC (Ruiz-Loredo et al., 2012). Here, we investigated the role of PKC in small airway contraction by characterizing its contribution to: (a) the contractile response, (b) Ca2+ signaling in SMCs, (c) changes in SMC Ca2+ sensitivity, and (d) the cellular mechanisms underlying Ca2+ oscillations and Ca2+ sensitization. We found that PKC activation induced airway SMC twitching that correlated with low frequency Ca2+ oscillations. These Ca2+ oscillations were generated by repetitive intracellular Ca2+ release through RyR after PKC-induced activation of LVGCs. PKC activation also led to phosphorylation of CPI-17 and rMLC, suggesting that MLCP inhibition mediates PKC-induced Ca2+ sensitization. Finally, activation of PKC with phorbol esters or exposure of lung slices to thrombin increased the contractile response of the airways to membrane depolarization.
MATERIALS AND METHODS
Most reagents were obtained either from Invitrogen, Life Technologies, and Gibco, or Sigma-Aldrich. The PKC activators PMA and phorbol 12,13-dibutyrate (PDBu) were purchased from LC Laboratories. GF-109203X and cyclopiazonic acid (CPA) were from Enzo Life Sciences, whereas Y-27632, nifedipine, and ryanodine were from Abcam. Thrombin, purified from bovine plasma, was from Sigma-Aldrich. Hanks’ balanced salt solution was prepared from a 10× stock solution (Invitrogen), supplemented with 20 mM HEPES buffer, and adjusted to pH 7.4 (sHBSS). Ca2+-free sHBSS was prepared from a 10× Ca2+- and Mg2+-free stock solution (Invitrogen) and supplemented with 20 mM HEPES, pH 7.4, 0.9 mM MgCl2, and 1.0 mM EGTA. PMA and PDBu were prepared at 20 and 10 mM, respectively, in DMSO. Stock solutions were diluted in sHBSS to the final concentration on the same day of use; the concentration of vehicle (DMSO) never exceeded 0.1%.
Preparation of lung slices
Mouse lung slices were prepared as described previously (Perez and Sanderson, 2005; Perez-Zoghbi et al., 2010) with some modifications. 8–12-wk-old male C3H inbred mice (Charles River) were killed with IP sodium pentobarbital (40 mg/Kg) and transferred to a clean dissection board in a dedicated sterile environment. Each animal was rinsed with 70% EtOH, the chest cavity was opened, and the trachea was exposed and cannulated with an intravenous (IV) catheter tube containing two input ports (20G Intima; BD), which was secured in position using tape. The lungs were inflated with 1.4 ± 0.1 ml of 2% agarose (low melting temperature agarose; USB Corporation) in sHBSS, using a syringe attached to one port of the IV catheter, and subsequently with ∼0.2 ml of air, using another syringe attached to the second port. The air injection was used to flush the agarose out of the airways and into the distal alveolar space. The agarose was gelled by cooling the lungs with a cotton ball soaked in ice-cold sHBSS and maintaining the mouse body at 4°C for 20 min. Lungs and heart were removed from the animal and held in ice-cold sHBSS for 15 min. Lung lobes were separated and trimmed near the main bronchus to create a base. Each lobe was transferred to the specimen syringe tube of a tissue slicer (Compresstome VF-300; Precisionary Instruments), with the lobe base sitting on the tube. The lung lobe was embedded first into ∼1 ml of 2% agarose and then fully covered with 6% gelatin. After the agarose and gelatin solidified, the block was cut into serial sections of 140 µm (or 220 µm for Western blot experiments), starting at the peripheral edge of the lung lobe. The entire procedure was performed in a safety cabinet under sterile conditions. Lung slices were observed on an inverted phase-contrast microscope and checked for the presence of airways. The first few slices usually lacked well-defined airways and were discarded. The subsequent 15–20 slices containing small terminal airways were collected and stored briefly in sHBSS. The slices were then incubated in low glucose Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 1× antibiotic solution containing l-glutamine, penicillin, and streptomycin (Invitrogen) and 50 µg/ml gentamicin (Sigma-Aldrich) at 37°C and 10% CO2 in a cell culture incubator. We used lung slices maintained for up to 48 h; no significant changes in airway contractility in response to 0.5 µM 5-HT or 0.5 µM ACh were detected during this period. For experiments, we only used lung slices that contained airways with a lumen diameter of 100–300 µm, completely lined by active ciliated epithelial cells, and fully attached to the surrounding lung parenchyma. The Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee approved our animal studies.
Measurement of airway contraction
Lung slices were mounted at the center of a 22 × 40–mm cover glass in a custom-made perfusion chamber and held in place with a small sheet of nylon mesh. A small hole was cut in the mesh and centered over the selected airway. A second 11 × 30–mm cover glass, edged with silicone grease, was placed over the mounted lung slice to create a thin rectangular chamber. The lung slice was perfused by adding solution at one end of the chamber and removing it by suction at the opposite end by means of a gravity-fed, computer-controlled perfusion system. The volume of the chamber (∼100 µl) and the perfusion rate (∼800 µl/min) were kept constant for the duration of each experiment. The chamber was placed on the stage of an inverted phase-contrast microscope (Diaphot TMD; Nikon), and lung slices were imaged with a 10× objective. Digital images (640 × 488 pixels) were recorded to a hard drive in time-lapse (0.5 Hz) using a CCD camera (KP-M1A; Hitachi), frame grabber (Picolo; Euresys), and image acquisition software (Video Savant; IO Industries). The area of the airway lumen was calculated from each image using a custom-written script in Video Savant, allowing us to distinguish the lumen from the surrounding tissue by means of a series of image-processing functions including filtering, smoothing, grayscale thresholding, and binarization, followed by calculation of the lumen cross-sectional area by pixel summation. The lumen area was normalized to the area before stimulation using Excel (Microsoft), and the changes in lumen area were plotted versus time using Origin software (Microcal). Line-scan analysis of images was performed by extracting a line of pixels from each image and placing them sequentially to form a time sequence in a single image. All experiments were performed at room temperature.
Measurements of intracellular Ca2+
Approximately 10–12 lung slices were incubated for 50 min at 30°C in 2 ml sHBSS supplemented with 20 µM Oregon green 488 BAPTA-1 acetoxymethyl ester (Invitrogen) dissolved in 20 µl of dry DMSO plus 5 µl of 20% Pluronic F-127 (Sigma-Aldrich) in DMSO. Slices were then transferred to 2 ml sHBSS and incubated for 50 min at 30°C to allow for de-esterification of the acetoxymethyl group. Lung slices were mounted in the perfusion chamber as described previously, and fluorescence imaging was performed with a custom-made video-rate confocal microscope (Sanderson and Parker, 2003). The sample was illuminated with a 488-nm laser beam, and fluorescence emission (510–530 nm) was collected with a photomultiplier tube (PMT C7950; Hamamatsu Photonics) and frame grabber (Alta-AN; BitFlow, Inc.). Images were recorded at 15 Hz using Video Savant. Changes in fluorescence intensity were analyzed by selecting regions of interest (ROI) ranging from 25 to 49 pixels2. Average fluorescence intensities of an ROI were obtained, frame-by-frame, using a custom-written script designed to track the ROI within an SMC as it moved with contraction. Final fluorescence values were expressed as a fluorescence ratio (F/F0) normalized to the initial fluorescence (F0). All experiments were performed at room temperature.
Preparation of Ca2+-permeabilized lung slices
To clamp the [Ca2+]i, airway SMCs were made permeable to external Ca2+ without detergents or toxins, using a technique that does not damage the cell membrane but instead exploits endogenous Ca2+-permeable ion channels (see Bai and Sanderson, 2006b, and Perez-Zoghbi and Sanderson, 2007, for full details and validation). Lung slices were exposed to 20 mM caffeine and 25 µM ryanodine for 4 min, followed by a washout with sHBSS for 10 min. This treatment locks the RyR in the SR of airway SMCs in an open state, thereby depleting intracellular Ca2+ stores and, consequently, increasing Ca2+ influx across the plasma membrane through store-operated channels. In these caffeine- and ryanodine-treated lung slices, the [Ca2+]i of airway SMCs is proportional to the extracellular Ca2+ concentration ([Ca2+]e). We exposed lung slices to sHBSS containing 1.3 mM Ca2+, thereby clamping [Ca2+]i at a high concentration. In all of these experiments, [Ca2+]i was monitored by confocal microscopy and did not change during the addition of experimental compounds.
Measurement of CPI-17 phosphorylation by Phos-tag SDS-PAGE and Western blot
For these analyses, we used lung slices that contained two or three well-preserved airways and no accompanying arteries and veins. The SMCs in these lung slices were almost exclusively airway SMCs, as confirmed by immunofluorescence with antibodies directed against SMC α actin (see below). Three slices per sample were washed with sHBSS and incubated with the indicated drugs for the indicated times. Subsequently, the slices were transferred to 1.2-ml tubes (Eppendorf) containing 100 µl of stimulation solution and quickly frozen by adding 300 µl of dry ice-cold acetone supplemented with 10% TCA and 10 mM DTT. Samples were maintained at −20°C overnight before protein extraction. The next day, the samples were sonicated (three cycles) and then centrifuged in Eppendorf tubes at 13,000 g for 10 min at 4°C. The supernatant was discarded, and the pellets were washed two to three times with 400 µl of cold acetone (−20°C) containing 10 mM DTT to remove residual TCA. The remaining pellet was air dried, suspended in 50 µl Laemmli buffer (Bio-Rad Laboratories), and boiled for 10 min at 95°C. Samples were loaded (25 µl/well) in a 15% SDS/PAGE gel supplemented with 50 µM Phos-tag ligand (AAL-107; NARD Institute, Ltd.) and two equivalents of MnCl2 according to the manufacturer’s instructions. Electrophoresis was run at 120 V for 90 min. After electrophoresis, the gel was washed for 15 min with transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol, pH 8.3) supplemented with 100 mM EDTA (to remove the Mn2+) and then with transfer buffer without EDTA (twice for 5 min each). Proteins were transferred electrophoretically to a PDVF membrane (0.2-µM pore size; Bio-Rad Laboratories) using a Mini-Trans-Blot Cell (Bio-Rad Laboratories) for 1.5 h at 100 V and 4°C. Immediately after protein transfer, the membrane was washed with PBS for 5 min followed by incubation in 4% paraformaldehyde (Sigma-Aldrich) in PBS for 45 min. This procedure reduces protein loss during subsequent Western blot steps and improves the efficiency of protein detection (Takeya et al., 2008). Subsequently, the membrane was blocked with 5% Non-Fat Dry Milk (Bio-Rad Laboratories) for 1 h and exposed overnight to the following primary antibodies, all diluted in blocking solution: mouse monoclonal anti-SMC α actin antibody (diluted at 1:3,000; Santa Cruz Biotechnology, Inc.); goat polyclonal anti–CPI-17 antibody (diluted at 1:500; Santa Cruz Biotechnology, Inc.); rabbit polyclonal anti-rMLC antibody (diluted at 1:500; Santa Cruz Biotechnology, Inc.); and rabbit polyclonal anti-phospho(ser19)-rMLC antibody (diluted at 1:200; Cell Signaling Technology). Subsequently, the membranes were washed and exposed for 6 h to the secondary antibodies: goat anti–mouse IgG conjugated with IR-Dye 680, donkey anti–goat IgG conjugated with IR-Dye 800 CW, or donkey anti–rabbit IgG conjugated with IR Dye 800 CW (LI-COR Biosciences). Bands were detected and quantified using an infrared imaging system (Odyssey; LI-COR Biosciences) and pseudocolored in red (680 nm) and green (800 nm). Phosphorylation of CPI-17 and rMLC was determined from the integrated band intensities of unphosphorylated (U) and phosphorylated (P) CPI-17 or rMLC bands according to the following relationship: (R = (P/(U + P)) × 100). For rMLC, the mono- and bi-phosphorylated bands (labeled 1P-rMLC and 2P-rMLC, respectively) were added to give total phosphorylated rMLC. Phosphorylation of rMLC at serine 19 was quantified as the ratio calculated from the integrated band intensities of total phosphorylated rMLC (probed with the anti-phospho(ser19) antibody) divided by the α-actin band.
Statistics
Statistical values are expressed as mean ± SE. Student’s t test was used to evaluate the significance between means.
Online supplemental material
Two videos consisting of sequences of phase-contrast or confocal fluorescence images were produced with “Video Savant” and are available at http://www.jgp.org/cgi/content/full/jgp.201210876/DC1.
RESULTS
Phorbol esters induced airway SMC twitching
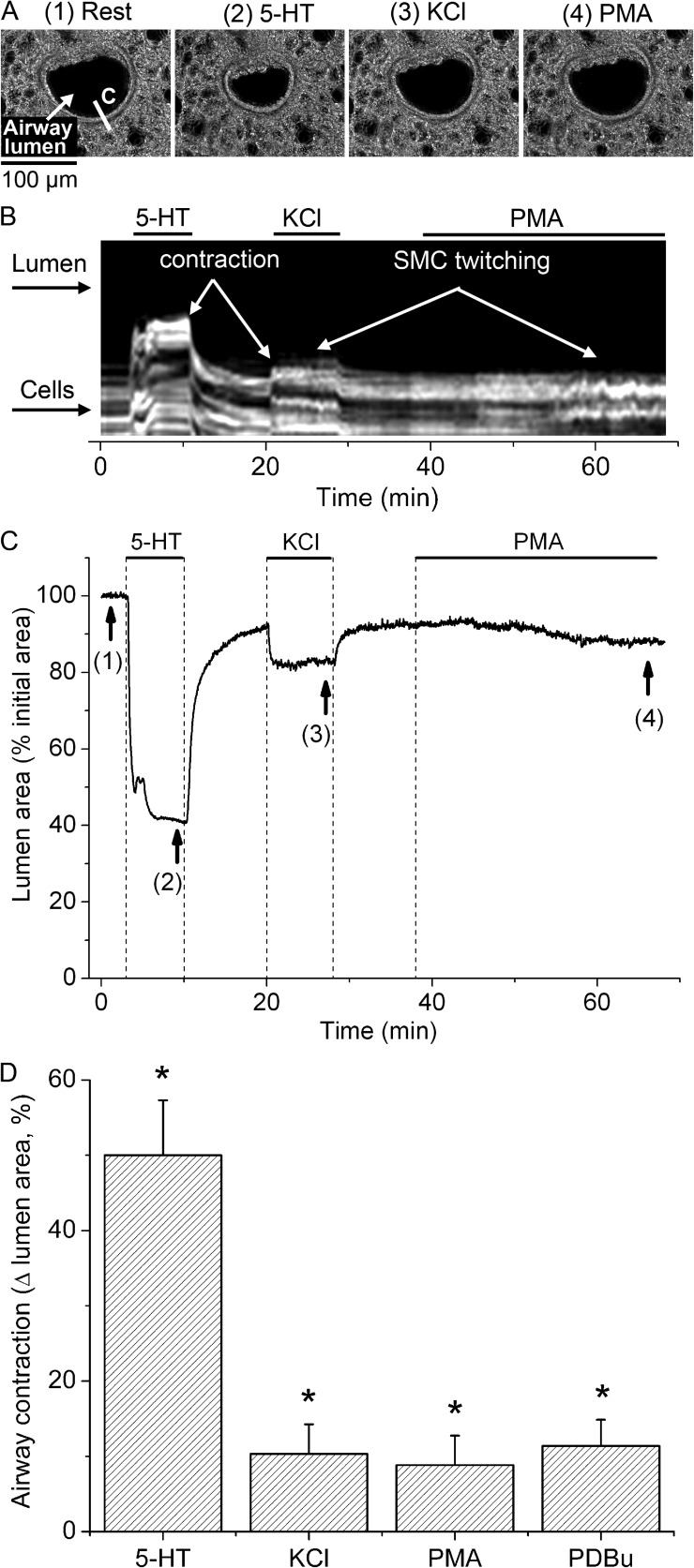
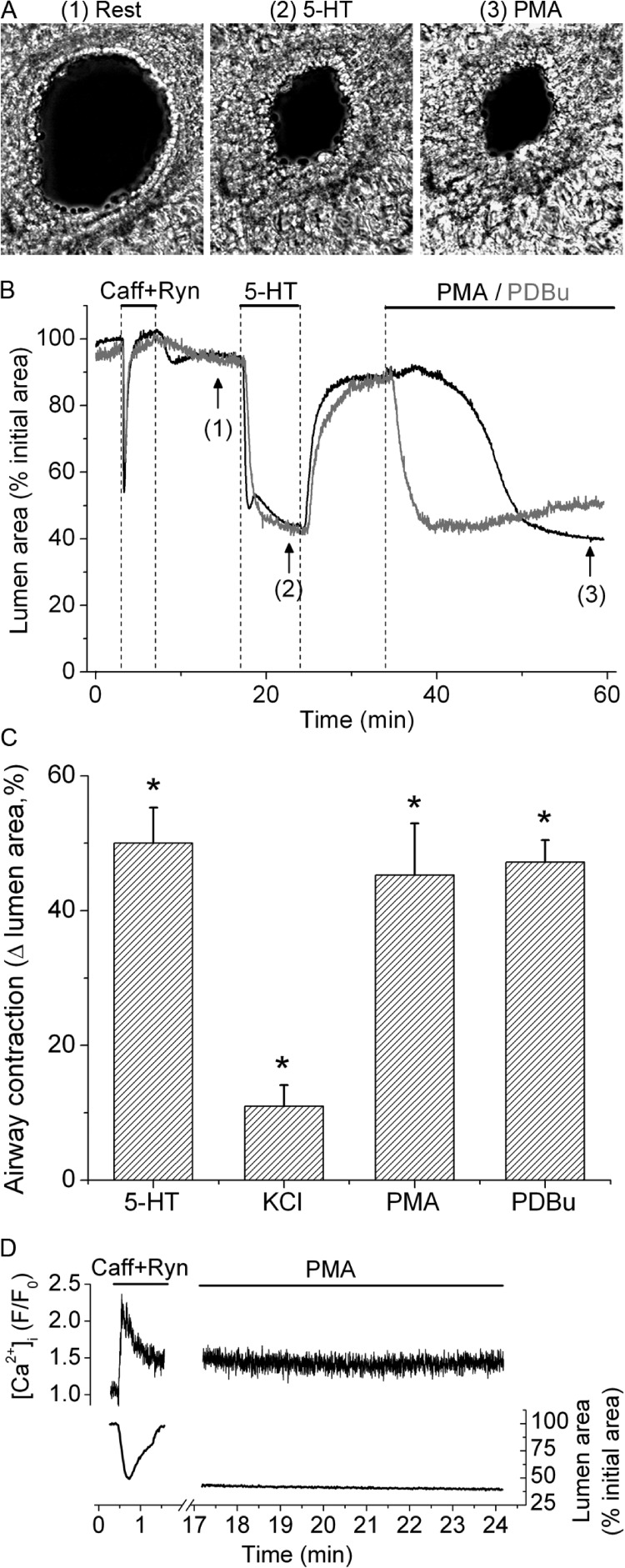
We characterized the contractile response of SMCs in small airways to PKC activation with phorbol esters (PMA and PDBu) and compared it to the responses to 5-HT and plasma membrane depolarization with KCl (Fig. 1). A typical lung slice with a small airway in cross section before (rest) and after sequential stimulation with 5-HT, KCl, and PMA is shown in Fig. 1 A. Superfusion of such lung slices with 10 µM PMA elicited transient and asynchronous contractions of the SMCs surrounding the airway lumen (SMC twitching) (Fig. 1 B and Video 1). This PMA-elicited SMC twitching was accompanied by a small, slow decrease in airway lumen area (Fig. 1 C) that was equivalent to 8.8 ± 3.9% of the total lumen area (mean ± SEM; Fig. 1 D) at 30 min of PMA stimulation. 1 µM PDBu also elicited SMC twitching and a small decrease in lumen area (11.4 ± 3.5%; Fig. 1 D); however, the onset of SMC twitching was faster for PDBu (4.4 ± 1.5 min) than for PMA (18 ± 4.7 min). These PMA and PDBu responses persisted for up to 1 h after the phorbol esters were washed out by superfusion with sHBSS. Stimulation with 50 mM of isosmotic, KCl-containing sHBSS also resulted in SMC twitching and a small decrease in airway lumen area (10.9 ± 3.9%). In contrast, stimulation with 0.5 µM 5-HT produced a much larger decrease in airway lumen area (49.7 ± 7.3%) without initiating SMC twitching. Airway responses to KCl and 5-HT were rapidly reversed by washout with sHBSS. These results suggest that PKC activation induces SMC twitching in small airways.
Figure 1.
Contractile response of airway SMCs to 5-HT, KCl, and phorbol esters. (A) Representative phase-contrast images showing an airway before stimulation (1) and after stimulation with 0.5 µM 5-HT (2), 50 mM of isosmotic KCl sHBSS (3), and 10 µM PMA (4), taken at the times indicated by arrows and corresponding numbers in the trace shown in C. (B) Line scan obtained from phase-contrast images at the region indicated by a line on image 1 in A, showing the movement of a few cells in the periphery of the airway wall in response to stimulation. 5-HT induced a strong and sustained movement of cells (light gray lines) toward the airway lumen (black), whereas KCl and PMA induced transient cell movements (SMC twitching, observed as striated white lines) and small sustained cell movements. (C) Trace showing the changes in total airway lumen cross-sectional area during superfusion with 5-HT, KCl, and PMA (upper lines). Washout of stimuli was performed by superfusion of lung slices with sHBSS. Sustained airway contraction induced by PMA was small and developed slowly as compared with airway contractions induced by 5-HT and KCl. (D) Summary of sustained airway contraction measured as the decrease in lumen area at 8 min after the addition of 5-HT or KCl, or at 30 min after the addition of PMA or 5 µM PDBu. Data are means ± SEM of seven airways (each from a different lung slice) from three mice. *, different from baseline airway lumen area (P < 0.01). A time-lapse movie showing the airway responses to 5-HT, KCl, and PMA from the representative experiment presented here is shown in Video 1.
Phorbol esters induce Ca2+ oscillations in airway SMCs
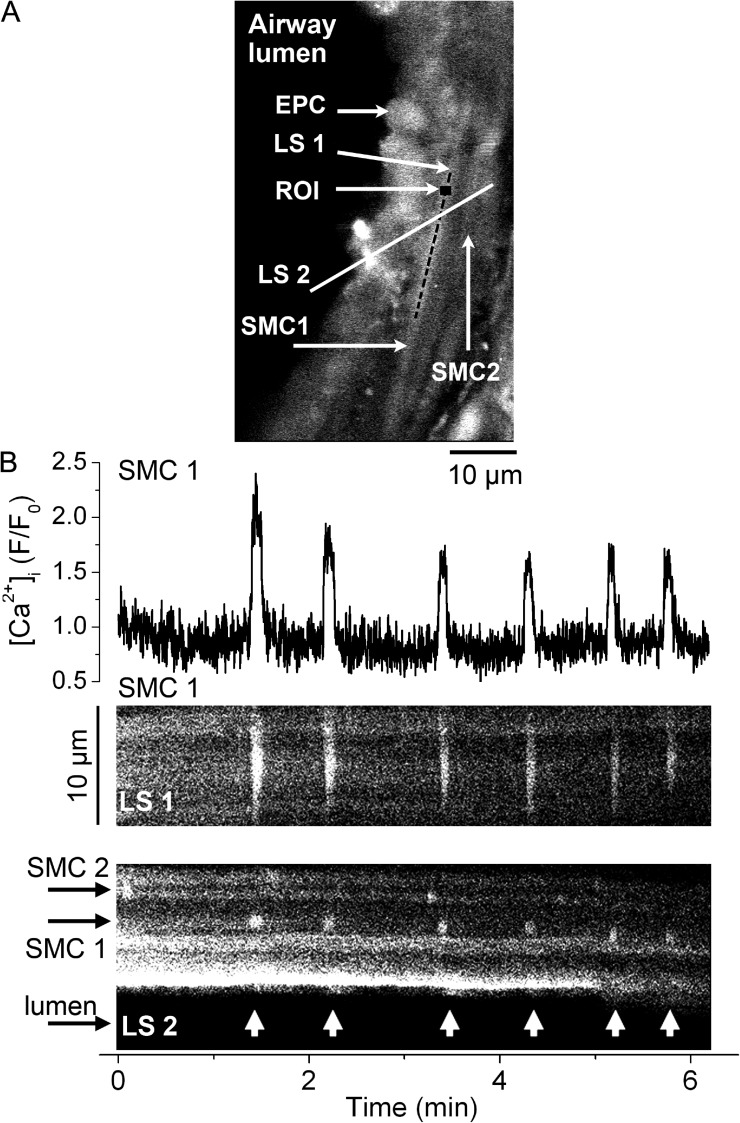
We next characterized the Ca2+ signals associated with airway SMC twitching induced by phorbol esters. We assessed [Ca2+]i in airway SMCs in lung slices using confocal video microscopy (Fig. 2 A). Stimulation with PMA induced Ca2+ oscillations in airway SMCs (Fig. 2 B). These Ca2+ oscillations started after ∼20 min of PMA superfusion and always persisted for several minutes (up to at least 1 h) independently of whether PMA was continuously being superfused or if it was removed by superfusion with sHBSS. PMA induced low frequency Ca2+ oscillations (1.6 ± 1.1 cycles/min; eight SMCs from six slices from three mice) that showed little change in frequency for several minutes. They propagated along the long axis of the SMC as a Ca2+ wave (Fig. 2 B, LS1, and Video 2) with an average velocity of 27.6 ± 4.3 µm/s. Each Ca2+ oscillation was characterized by a fast increase in Ca2+ (0.8 ± 0.3 s to peak), a relatively wide peak (4.9 ± 0.4 s), and a fast decrease to basal [Ca2+]i (1.4 ± 0.5 s). Ca2+ oscillations in neighboring SMCs were unsynchronized and occurred at different frequencies (Fig. 2 B, LS2, and Video 2). These Ca2+ oscillations were associated with transient SMC contractions that caused airway cells to move transiently toward the airway lumen (SMC twitching; Fig. 2 B, LS2). 1 µM PDBu activated similar Ca2+ oscillations in airway SMCs with a frequency of 2.4 ± 1.5 cycles/min; however, these Ca2+ oscillations started at ∼5 min of PDBu superfusion. These results indicate that phorbol esters induce low frequency Ca2+ oscillations in airway SMCs that appear to be synchronized with SMC twitching.
Figure 2.
Simultaneous Ca2+ signaling and contraction in airway SMCs induced by phorbol esters. (A) Fluorescence confocal image of an airway region in a lung slice showing epithelial cells (EPC) lining the airway lumen (left, black area) and the underlying SMCs (SMC1 and SMC2). A small (7 × 7–pixel) ROI within SMC1 and two lines (LS1 and LS2) indicate the areas selected for the representative fluorescence trace and line scans presented in B. (B; top) Fluorescence (F/F0) trace showing the Ca2+ oscillations stimulated by 10 µM PMA (added 30 min before the recording started) in a single airway SMC. (B, middle) Line-scan analysis from the longitudinal axes of the SMC1 (LS1) shows the propagation of the Ca2+ oscillations along the SMC as Ca2+ waves (near-vertical white lines). (B; bottom) Line-scan analysis from an area (LS2) across SMC1, SMC2, epithelial cells and part of the airway lumen shows the Ca2+ oscillations in the SMCs (short vertical white lines) and transient displacements of the epithelial cells toward the airway lumen (arrow heads) caused by SMC twitching occurring concomitantly with the Ca2+ oscillations in SMC1. Data are representative of six experiments in lung slices from three mice. A time-lapse movie showing the Ca2+ oscillations stimulated by PMA in airway SMCs is shown in Video 2.
Pharmacological inhibition of PKC blocks PMA-induced Ca2+ oscillations and SMC twitching
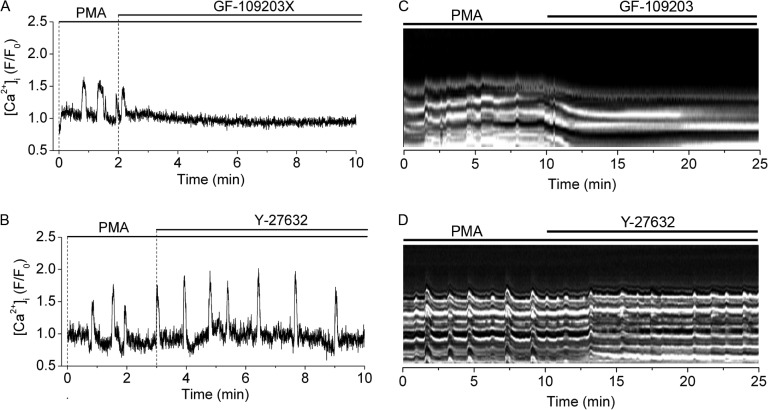
Next, we determined the effect on phorbol ester–induced Ca2+ oscillations and SMC twitching of the PKC inhibitor GF-109203X and the ROK inhibitor Y-27632. Ca2+ oscillations and SMC twitching induced by PMA were rapidly and completely blocked by 1 µM GF-109203X but not by 10 µM Y-27632 (Fig. 3). These results further suggest that PMA-induced Ca2+ oscillations and SMC twitching depend on PKC activation and indicate that they are independent of ROK activity.
Figure 3.
Sensitivity of PMA-induced Ca2+ oscillations and SMC twitching to inhibition of PKC or ROK. The effect of PKC inhibitor GF-109203X (1 µM) or ROK inhibitor Y-27632 (10 µM) on (A and B) Ca2+ oscillations in single airway SMCs and (C and D) SMC twitching in the airway wall induced by 10 µM PMA (added 30 min before the recordings started). Line scans showing SMC twitching were obtained from a small region on the airway wall from phase-contrast images (as illustrated in Fig. 1). PMA-induced Ca2+ oscillations and SMC twitching (striated white lines) were inhibited (straight white lines) by GF-109203X but not by Y-27632. Each experiment is representative of six airways in lung slices from three mice.
PMA-induced Ca2+ oscillations and SMC twitching depend on Ca2+ entry and Ca2+ release
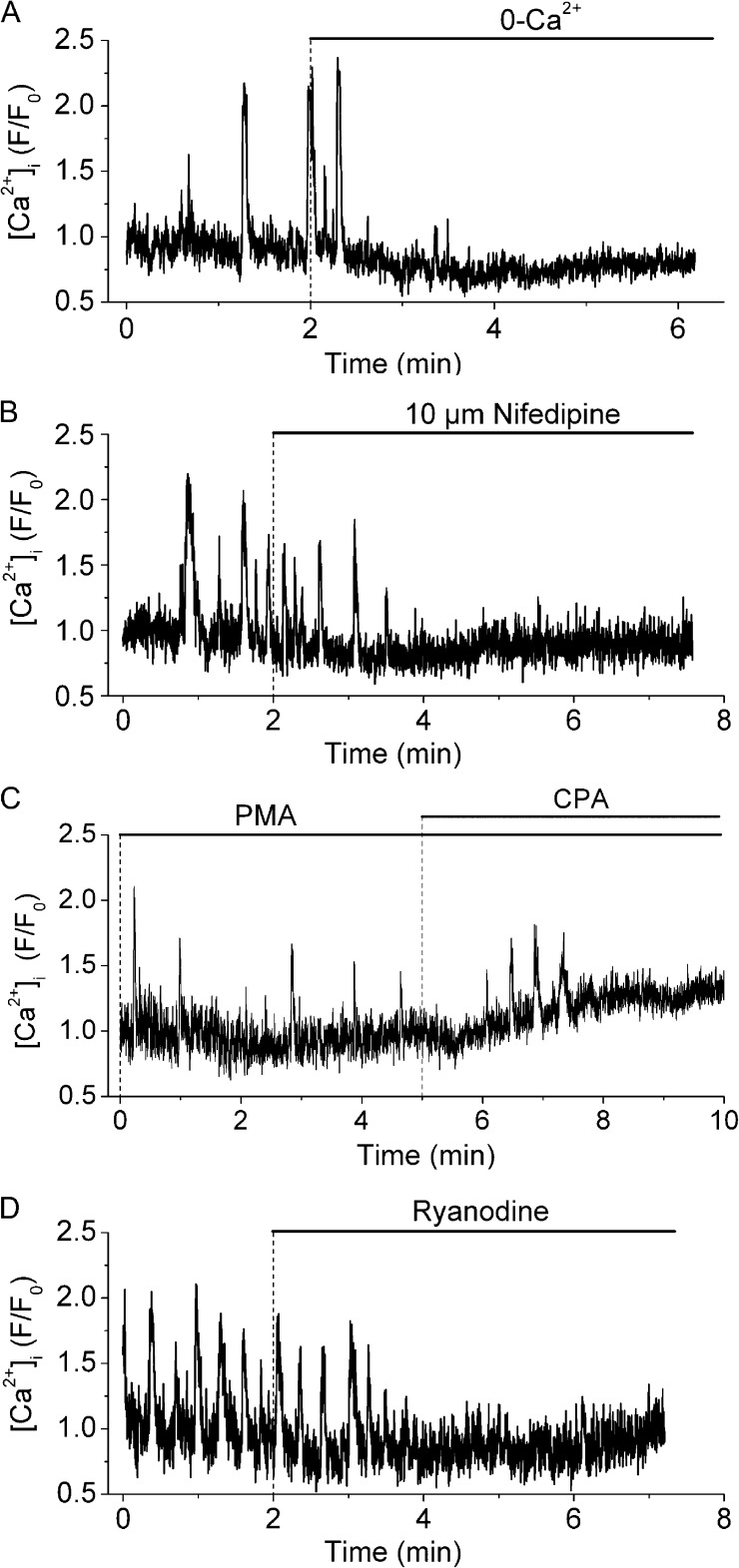
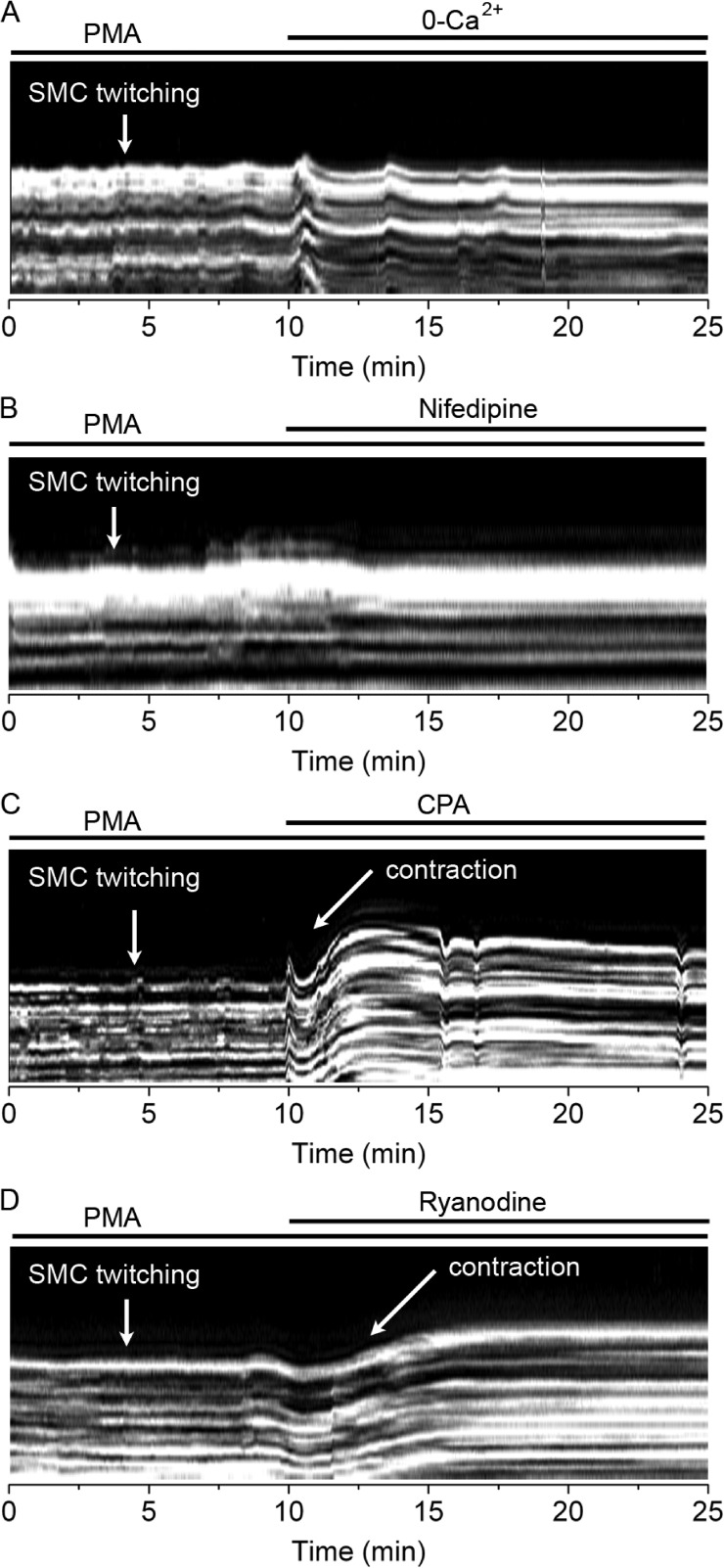
Next, we studied the sensitivity of PMA-induced Ca2+ oscillations to manipulations that inhibit either Ca2+ entry across the plasma membrane or Ca2+ release from internal stores (Fig. 4). Removal of extracellular Ca2+ or exposure to the LVGC blocker nifedipine (10 µM) rapidly blocked PMA-induced Ca2+ oscillations (Fig. 4, A and B), suggesting that PMA-induced Ca2+ oscillations depend on Ca2+ entry through LVGCs. The sarcoplasmic/endoplasmic reticulum Ca2+ ATPase inhibitor CPA (10 µM) elicited a slow block of the Ca2+ oscillations (Fig. 4 C), suggesting that Ca2+ oscillations also depend on intracellular Ca2+ stores. CPA also induced a sustained increase in [Ca2+]i, indicating that it caused a depletion of the Ca2+ stores, which presumably stimulated Ca2+ influx through store-operated Ca2+ channels. PMA-induced Ca2+ oscillations were also inhibited by 25 µM ryanodine (Fig. 4 D), indicating that they depended on Ca2+ release through RyRs. Collectively, these results suggest that PKC promotes LVGC activation in airway SMCs, leading to Ca2+ influx into the cytosol followed by its accumulation into and overloading of intracellular Ca2+ stores. This leads to repetitive cycles of Ca2+ release through RyR and the generation of Ca2+ oscillations that propagate along the SMC cytosol as Ca2+ waves.
Figure 4.
Effect of extracellular Ca2+ removal, nifedipine, CPA, and ryanodine on PMA-induced Ca2+ oscillations. Ca2+ oscillations in single airway SMCs induced by 10 µM PMA (added 30 min before the recording started) and its inhibition by: (A) superfusion with Ca2+-free sHBSS, (B) 10 µM nifedipine, (C) 10 µM CPA, or (D) 25 µM ryanodine. Each trace is representative of four experiments in lung slices from three mice.
Because PMA-induced Ca2+ oscillations and SMC twitching were synchronous, we determined how manipulations that inhibit the Ca2+ oscillations affect SMC twitching. We used phase-contrast microscopy for these studies, which allowed us to record SMC twitching for a longer time period. PMA-induced SMC twitching was fully inhibited by removal of extracellular Ca2+ or exposure to nifedipine (Fig. 5, A and B), whereas CPA and ryanodine not only blocked PMA-induced SMC twitching but also elicited a sustained airway contraction (Fig. 5, C and D). The ryanodine-induced contraction was not reversible after 10 min of washout (n = 3 experiments from two mice), consistent with the slow dissociation kinetics of ryanodine from the RyR (Williams and Tanna, 2004).
Figure 5.
Effect of extracellular Ca2+ removal, nifedipine, CPA, and ryanodine on PMA-induced airway SMC twitching. Line scans from airway wall regions obtained from phase-contrast images (similar to that presented in Fig. 1) showing airway SMC twitching induced by 10 µM PMA (added 30 min before the recordings started) and their sensitivity to: (A) Ca2+-free sHBSS, (B) 10 µM nifedipine, (C) 10 µM CPA, or (D) 25 µM ryanodine. PMA-induced SMC twitching was inhibited by removal of extracellular Ca2+, nifedipine, CPA, and ryanodine. Each line scan is representative of three experiments in lung slices from two mice.
Phorbol esters increased Ca2+ sensitivity of SMC contraction in small airways
To determine whether PKC activity affects the Ca2+ sensitivity of SMC contraction in small airways, we assessed the effects of phorbol esters on airway contraction in Ca2+-permeabilized airway SMCs (Fig. 6). We exposed lung slices to 20 mM caffeine and 25 µM ryanodine for 3 min and then superfused them with sHBSS containing our standard Ca2+ concentration ([Ca2+] = 1.3 mM). This treatment leads to depletion of Ca2+ stores, activation of store-operated Ca2+ entry, and a sustained increase in [Ca2+]i that is maintained for more than 1 h after washout of caffeine and ryanodine (Perez-Zoghbi and Sanderson, 2007; Perez-Zoghbi et al., 2010). As expected, the addition of 5-HT to Ca2+-permeabilized lung slices induced a strong contraction of the airway, with the subsequent washout accompanied by relaxation to the initial lumen area (Fig. 6, A and B). This agonist-induced contraction occurred in the presence of a constant [Ca2+]i, demonstrating agonist-induced Ca2+ sensitization (Perez-Zoghbi and Sanderson, 2007; Perez-Zoghbi et al., 2010). Subsequent stimulation with 10 µM PMA or 1 µM PDBu induced a contraction similar in magnitude to that induced by 5-HT (Fig. 6, B and C), whereas KCl-induced contraction, although significant, was smaller (Fig. 6 C). In Ca2+-permeabilized lung slices, PMA-induced airway contraction occurred in the absence of changes in [Ca2+]i or Ca2+ oscillations (Fig. 6 D). These results suggest that PKC activation by phorbol esters induces strong Ca2+ sensitization in airway SMCs.
Figure 6.
Ca2+ sensitization induced by agonists and phorbol esters. Ca2+ sensitization of airway SMCs studied in Ca2+-permeabilized lung slices with 20 mM caffeine plus 25 µM ryanodine. (A) Representative phase-contrast images of a Ca2+-permeabilized lung slice showing an airway before (1) and after stimulation with 5-HT (2) or PMA (3) at the times indicated by the corresponding numbers in B. (B) Traces from two similar experiments showing the changes in airway lumen area during perfusion of caffeine plus ryanodine, 0.5 µM 5-HT, and 10 µM PMA (black trace) or 1 µM PDBu (gray trace) at the times indicated by the upper lines. (C) Summary of airway contractions induced by 5-HT, 50 mM of isosmotic KCl, PMA, or PDBu (mean ± SEM; eight airways from slices from four mice) in Ca2+-permeabilized lung slices obtained from experiments similar to that shown in B. *, different from baseline airway lumen area (P < 0.01). (D) Simultaneous changes in SMC [Ca2+]i (top trace) and airway lumen area (bottom trace) during stimulation with caffeine plus ryanodine and after subsequent addition of PMA (added 20 s after washout of caffeine plus ryanodine). Traces in D are representative of four lung slices from three mice.
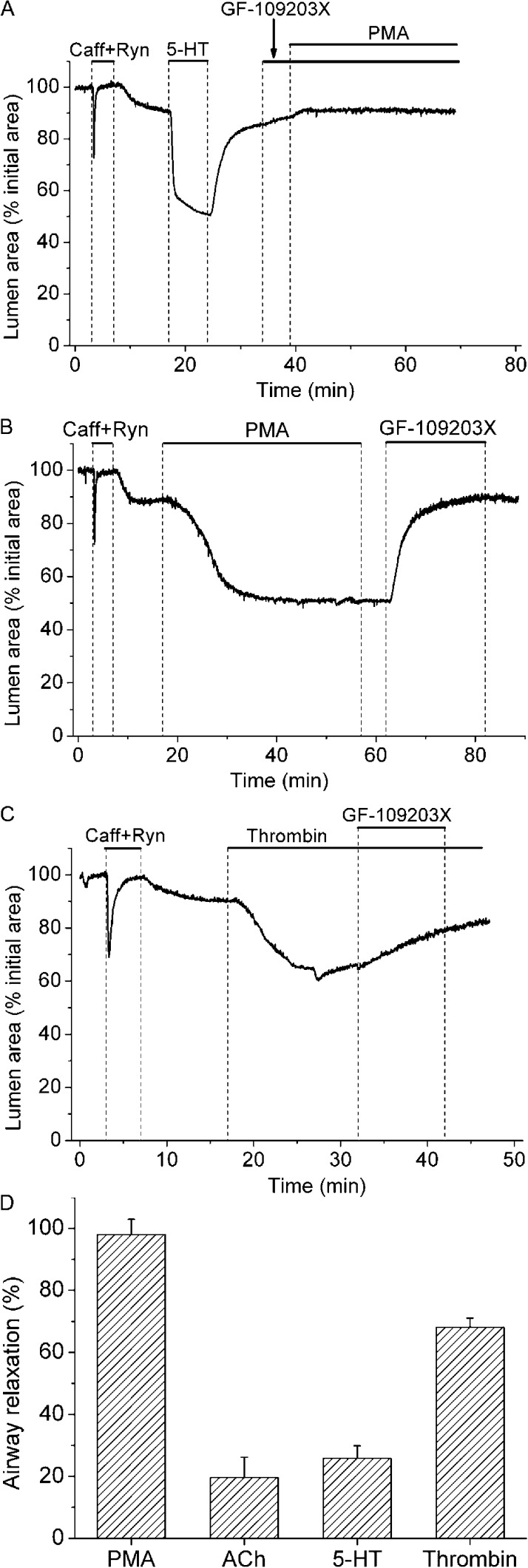
The role of PKC in Ca2+ sensitization induced by PMA and contractile agonists
We used caffeine- and ryanodine-treated lung slices and the PKC inhibitor GF-109203X to evaluate the role of PKC in Ca2+ sensitization induced by PMA or by contractile agonists with physiological or pathophysiological roles in the lung (Fig. 7). The addition of GF-109203X before (Fig. 7 A) or after (Fig. 7 B) PMA completely blocked PMA-induced airway contraction. Thrombin induced a strong airway contraction in Ca2+-permeabilized lung slices, indicating that thrombin causes Ca2+ sensitization in this system (Fig. 7 C); thrombin-induced airway contractions were attenuated by GF-109203X, suggesting that thrombin-induced Ca2+ sensitization was mediated, at least in part, by PKC. In contrast, 5-HT– and ACh-induced airway contractions were largely resistant to GF-109203X, suggesting that Ca2+ sensitization induced by 5-HT and ACh is not mediated by PKC (Fig. 7 D).
Figure 7.
Effect of PKC inhibitor GF-109203X on Ca2+ sensitization induced by PMA or contractile agonists. Lung slices were first exposed to 20 mM caffeine plus 25 µM ryanodine (upper bars) to induce Ca2+ permeabilization. (A) Representative experiment showing the effect of airway exposure to 1 µM GF-109203X before and during stimulation with 10 µM PMA. Contractile response of the airways after Ca2+ permeabilization was accessed with 0.5 µM 5-HT before their exposure to GF-109203X and PMA. This experiment is representative of six lung slices from three mice. (B and C) Airway contraction induced by PMA or 0.1 U/ml thrombin and the subsequent relaxation induced by GF-109203X. (D) Summary of the effect of GF-109203X on airway contraction in Ca2+-permeabilized airways precontracted with 10 µM PMA, 0.25 µM ACh, 0.5 µM 5-HT, and 0.1 U/ml thrombin. Airway relaxation (mean ± SEM; n = 5 lung slices from 3 mice) was obtained from experiments similar to that shown in B and C using the appropriated contractile agonist or PMA.
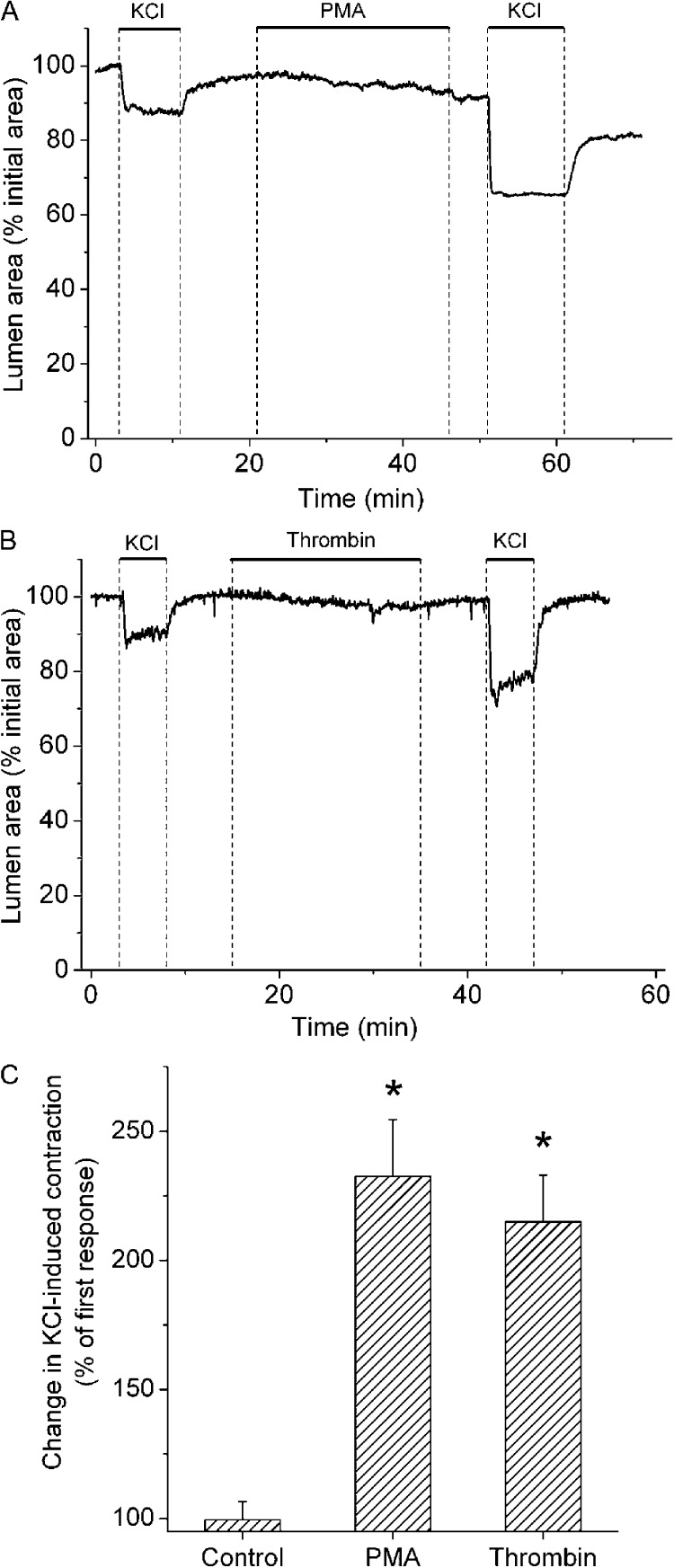
PMA and thrombin potentiated airway contraction induced by membrane depolarization with KCl
Because PMA and thrombin elicited PKC-mediated Ca2+ sensitization, we determined whether these agents increased the contractile response of airways to KCl-induced membrane depolarization. Both PMA and thrombin induced weak airway contractions in normal (nonpermeabilized) lung slices (Fig. 8); however, these agents more than doubled the contractile response to KCl. These results confirm that PKC activation with phorbol esters or thrombin induces Ca2+ sensitization in small airways, thereby potentiating the contractile response to stimuli that induce membrane depolarization and increase [Ca2+]i.
Figure 8.
Sensitization of KCl-induced airway contraction by PKC activation and thrombin. (A and B) Representative experiments showing the increase in KCl-induced airway contraction caused by the exposure of lung slices to 10 µM PMA or 0.1 U/ml thrombin, respectively. (C) Summary of effects of sHBSS (control), PMA, and thrombin on airway contraction induced by 50 mM of isosmotic KCl. The ratios were calculated by dividing the KCl-induced airway contractions after and before the treatments from experiments similar to those shown in A and B. *, significantly higher than the first stimulation (P < 0.01).
PKC activity induces phosphorylation of CPI-17 and increases phosphorylated rMLC in small airways
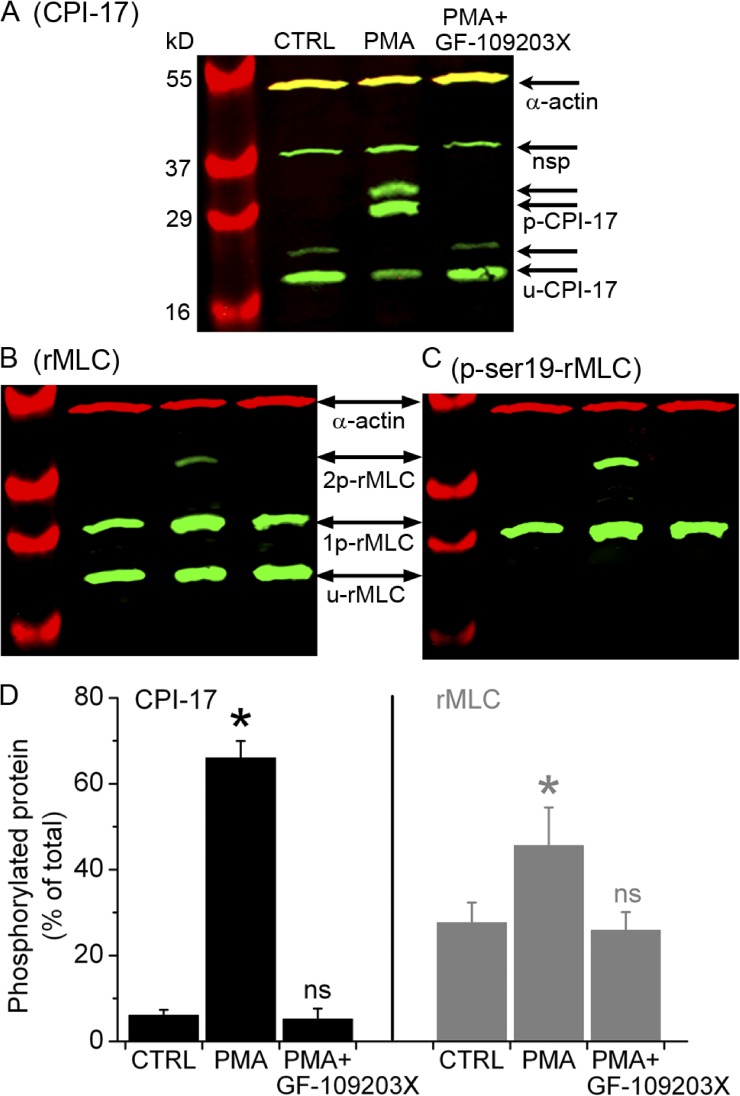
CPI-17, a specific target of PKC, is almost exclusively expressed in smooth muscle; when phosphorylated, CPI-17 inhibits MLCP (Wright et al., 2012). We used Western blot analysis to detect and measure phosphorylation of CPI-17 and rMLC in small airways. Samples were prepared from Ca2+-permeabilized lung slices (control) or Ca2+-permeabilized slices incubated with PMA and then either exposed to GF-109203X or left unexposed to this inhibitor (Fig. 9). Phosphorylated and unphosphorylated proteins were separated by Phos-tag SDS PAGE. Whereas CPI-17 was mostly unphosphorylated in control slices, PMA induced marked CPI-17 phosphorylation (Fig. 9, A and D). A similar increase in CPI-17 phosphorylation (72 ± 7%; three samples from two mice) was apparent in nonpermeabilized lung slices stimulated with PMA. The increase in CPI-17 phosphorylation in response to PMA was lost in slices subsequently exposed to GF-109203X. Stimulation with PMA also increased rMLC phosphorylation, and this was also reversed by GF-109203X (Fig. 9, B and C), although rMLC in unstimulated airways showed a relatively high level of basal phosphorylation (∼25% of total rMLC). Phosphorylation of rMLC at serine 19 (Fig. 9 C), calculated as the ratio between total phosphorylated rMLC (determined with antibody directed against phospho(ser19)-rMLC) and α actin, increased from 1.2 ± 0.2 in controls to 1.8 ± 0.3 in PMA-treated samples, indicating that PMA-induced rMLC phosphorylation occurred at serine 19. In addition, the absence of unphosphorylated rMLC bands in this last Western blot confirmed that the phosphorylated and unphosphorylated rMLC isoforms were correctly identified as labeled in Fig. 9 B. These results suggest that reversible phosphorylation of both CPI-17 and rMLC follows PKC activation in small airways.
Figure 9.
Phosphorylation of CPI-17 and rMLC induced by PMA and inhibited by GF-109203X. Separation of (A) phosphorylated (p-CPI-17) and unphosphorylated (u-CPI-17) CPI-17 and (B and C) mono-phosphorylated (1p-rMLC), bi-phosphorylated (2p-rMLC), and unphosphorylated (u-rMLC) rMLC by SDS-PAGE with polyacrylamide-bound phosphate-binding tag (Phos-tag SDS-PAGE) and detected by specific antibodies using Western blot. The Western blots in B and C were obtained after stripping previous antibodies and reprobing the membrane in A with antibodies directed against total rMLC and subsequently against phosphorylated rMLC at serine 19 (p-ser19-rMLC), respectively. Samples were prepared from selected lung slices that contained airways but not arteries and were incubated with 20 mM caffeine plus 25 µM ryanodine for 5 min, washed with sHBSS, and then incubated for 40 min with sHBSS (CTRL), 10 µM PMA, or PMA followed by 10-min exposure to 1 µM GF-109203X (upper labels). The antibody against CPI-17 detected two CPI-17 splice variants (arrow pairs) along with a nonspecific (nsp) band, as indicated by the manufacturer. Parallel samples were separated in an SDS-PAGE without Phos-tag to identify the phosphorylated/unphosphorylated CPI-17 and rMLC by verifying that each of these forms migrated in the same position in the gel without Phos-tag. SMC α actin was detected in all samples. (D) Ratio values (mean ± SEM) of p-CPI-17 to total CPI-17 and 1p+2p-rMLC to total rMLC from three experiments similar to that presented in A and B from three mice are shown.
DISCUSSION
We used phase-contrast video microscopy, confocal microscopy, Western blot analysis, and pharmacological activators and inhibitors to investigate the role of PKC in airway SMC contraction in lung slices and identify the mechanisms involved. We found that: (a) activation of PKC caused repetitive, unsynchronized, and transient contractions in the SMCs lining the airway lumen that resulted in a small decrease in airway luminal area; (b) this contractile activity correlated with low frequency Ca2+ oscillations in airway SMCs; (c) PKC activation with phorbol esters or thrombin elicited Ca2+ sensitization of SMC contraction and increased the contractile response of the airways to stimuli that increase Ca2; and (d) finally, PKC activation led to the reversible phosphorylation of CPI-17 and rMLC.
PKC activation induces small airway SMC twitching
Previous reports have shown that activation of PKC with phorbol esters (phorbol 12,13-diacetate, PMA, or PDBu) can have different effects on airway SMC tone depending on: (a) the animal species studied, (b) location in the airway tree, or (c) the presence or absence of stimuli that increase intracellular Ca2+ (such as KCl). For example, phorbol esters induce relaxation in guinea pig trachea rings (Huang et al., 1987; Souhrada and Souhrada, 1989; Morrison and Vanhoutte, 1991), initiate a biphasic response (a transient relaxation followed by a sustained contraction) in human bronchus (Yang and Black, 1995), and stimulate the contraction of isolated human bronchial SMCs (Rossetti et al., 1995). There are no significant contractile responses to phorbol esters in rat tracheal smooth muscle (Peiper et al., 1996) or mouse bronchial rings (Sakai et al., 2010). PMA-induced airway contraction is significantly increased by co-stimulation with KCl in all species studied, including mice and rats (Sakai et al., 2010). Our results in small airways show that exposure of mouse lung slices to phorbol esters (PMA and PDBu) induced transient and asynchronous small airway SMC contractions (SMC twitching) that resulted in a very small decrease in airway lumen area. Similarly, thrombin produced a small decrease in small airway lumen area. In accordance with Poiseuille’s law, airway resistance is inversely proportional to the square of the lumen cross-sectional area; therefore, even a small (∼9%) decrease in lumen area will lead to a significant (∼21%) increase in airway resistance. However, PKC activation or exposure to thrombin sensitized airway SMCs to Ca2+ and produced a marked potentiation of the contractile response of airways to stimuli that increase [Ca2+]i (see below).
Airway SMC twitching correlates with low frequency Ca2+ oscillations induced by phorbol esters
Several lines of evidence support the hypothesis that small airway contraction depends on the frequency of Ca2+ oscillations in SMCs. First, contractile agonists, including ACh or methacholine, 5-HT, and endothelin-1, induce a dose-dependent increase in the frequency of the Ca2+ oscillations, which positively correlates with airway contraction (Perez and Sanderson, 2005; Perez-Zoghbi and Sanderson, 2007). At maximal concentrations, these agonists induce high frequency Ca2+ oscillations (20–30 cycles/min) and strong airway contractions (50–80% reduction in airway lumen area). Second, cAMP-increasing agents, such as the β2 agonists isoproterenol, albuterol, or formoterol, or the adenylyl cyclase activator forskolin, decrease the frequency of Ca2+ oscillations induced by contractile agonists in rodent and human airways. This decrease in Ca2+ oscillation frequency is accompanied by airway relaxation in all cases (Bai and Sanderson, 2006a; Delmotte and Sanderson, 2008, 2010; Delmotte et al., 2010; Ressmeyer et al., 2010). Similarly, nitric oxide inhibits Ca2+ release through IP3 receptors, decreases the frequency of agonist-induced Ca2+ oscillations, and simultaneously causes airway relaxation (Perez-Zoghbi et al., 2010). Third, plasma membrane depolarization with KCl induces low frequency Ca2+ oscillations (one to two cycles/min) and weak airway contraction that is characterized by SMC twitching and a small (5–15%) decrease in lumen area (Perez and Sanderson, 2005). Here, we found that PKC activation with phorbol esters also induced low frequency Ca2+ oscillations (0.5–2.5 cycles/min) and airway SMC twitching with a small decrease in lumen area (5–10%). Collectively, these results indicate that low frequency Ca2+ oscillations in airway smooth muscle are associated with airway SMC twitching and support the hypothesis that airway SMC contraction is regulated by the frequency of Ca2+ oscillations.
The mechanism of PKC-induced Ca2+ oscillations
The low frequency Ca2+ oscillations induced by phorbol esters propagated along the longitudinal axes of the SMCs as Ca2+ waves. These Ca2+ oscillations were blocked by inhibition of the sarco-endoplasmic reticulum Ca2+ ATPase with CPA or by inhibition of RyRs with ryanodine, indicating that they were mediated by Ca2+ release through RyRs from intracellular stores. Similarly, previous studies have shown that the low frequency Ca2+ oscillations stimulated by KCl propagate as Ca2+ waves in airway SMCs, are blocked by CPA and ryanodine or tetracaine (another RyR inhibitor), and were suggested to be mediated by Ca2+ release via RyR (Perez and Sanderson, 2005; Bai et al., 2009). These studies also explored the mechanisms whereby KCl induces Ca2+ oscillations in airway SMCs. KCl stimulates Ca2+ influx in SMCs by inducing plasma membrane depolarization, thereby activating LVGCs. Accordingly, KCl-induced Ca2+ oscillations were inhibited by removal of extracellular Ca2+ or by LVGC blockers such as nifedipine and verapamil (Perez and Sanderson, 2005). In airway SMCs, Ca2+ influx through LVGCs could not be detected by Oregon green fluorescence as a steady increase in [Ca2+]i; rather, it was associated with repetitive, localized, and small Ca2+ transients that eventually generated Ca2+ waves. This activity was inhibited by CPA and ryanodine, suggesting that Ca2+ influx through LVGCs results in overloading of intracellular Ca2+ stores and the activation of Ca2+ release through RyR (Perez and Sanderson, 2005).
Here, we found that PKC-activated Ca2+ oscillations, like those induced by KCl, were inhibited by removal of extracellular Ca2+ or by nifedipine, suggesting that activation of PKC results in an initial increase in Ca2+ influx through LVGCs. Indeed, activation of LVGCs by PKC has been demonstrated in cardiac myocytes and in systemic vascular SMC preparations. For example, electrophysiological studies of mesenteric artery myocytes have shown that activation of PKC with PDBu increases LVGC conductance, decreases channel closing rate, and shifts the voltage dependence of channel opening to more negative potentials (Ren et al., 2010). Studies in rat cerebral arteries using total internal reflection fluorescence microscopy suggest that LVGCs organize in clusters (Navedo et al., 2005, 2006). In these studies, PKC activity was suggested to increase the opening of LVGCs in these clusters to induce localized Ca2+ influx events called “Ca2+ sparklets,” whereas protein phosphatase activity opposed LVGC opening. This PKC-regulated LVGC activity is thought to control the steady-state [Ca2+]i in these SMCs. Finally, LVGCs form molecular association with several PKC isoforms in cardiac myocytes, and channel activation may occur as a result of phosphorylation of a serine residue at the C terminus of the LVGC α subunit (Yang et al., 2005, 2009). Our results in small airways support the hypothesis that LVGC activity is regulated by PKC.
Phorbol esters and thrombin induce strong Ca2+ sensitization
Previous experiments with Ca2+-permeabilized lung slices suggest that mouse small airways have a low intrinsic (basal) Ca2+ sensitivity because they remain relaxed under conditions in which there is a sustained increase in SMC [Ca2+]i (Bai and Sanderson, 2006b, 2009; Perez-Zoghbi and Sanderson, 2007; Delmotte and Sanderson, 2010). The addition of agonists to these Ca2+-permeabilized lung slices elicits a strong airway contraction, indicating that they induce Ca2+ sensitization. Here, we show that phorbol esters induce a strong airway contraction in Ca2+-permeabilized lung slices. This contrasts with the SMC twitching and small decrease in lumen area stimulated by phorbol esters in nonpermeabilized lung slices and supports the hypothesis that airway contraction depends on both the [Ca2+]i and the degree of Ca2+ sensitization (Sanderson et al., 2008). Thus, to induce a strong sustained airway contraction, a stimulus must substantially increase both the [Ca2+]i and the Ca2+ sensitivity. Although phorbol esters strongly increased Ca2+ sensitivity, the low frequency Ca2+ oscillations they induce do not produce an increase in [Ca2+]i sufficient to induce a sustained airway contraction. Similarly, KCl induces low frequency Ca2+ oscillations, weak Ca2+ sensitization, and, as a result, SMC twitching and weak sustained contraction in mouse small airways.
Thrombin, a central protease in the coagulation cascade that is now recognized to contribute to asthma and other inflammatory lung diseases (de Boer et al., 2012), elicits modest airway and pulmonary vascular SMC contraction by stimulating proteinase-activated receptor 1 (Hauck et al., 1999; Maki et al., 2010). We found here that thrombin induced a weak airway contraction in nonpermeabilized lung slices. However, similar to phorbol esters, thrombin induced a strong airway contraction in lung slices with Ca2+-permeabilized SMCs, suggesting that it induces Ca2+ sensitization. Ca2+ sensitization by both phorbol esters and thrombin was also evident in nonpermeabilized lung slices, where both PMA and thrombin markedly potentiated the response to KCl. Consistent with our results, a potentiation of phorbol ester–induced airway contraction by KCl-induced membrane depolarization was reported previously (Sakai et al., 2010). We estimate that agonists that cause membrane depolarization will increase airway resistance four times more in airways that have been exposed to agents like thrombin that activate PKC than in airways that have not been exposed to such agents. Thus, activation of PKC in airway SMCs by molecules such as thrombin could sensitize the airways to contractile stimuli. Airways and alveoli are exposed to increased thrombin levels in asthma and other inflammatory lung diseases characterized by airway hyperresponsiveness (Gabazza et al., 1999; Wagers et al., 2004; Kanazawa and Yoshikawa, 2007), suggesting that these results may be physiologically significant.
The mechanism of Ca2+ sensitization and the role of CPI-17 phosphorylation
In many experimental models, Ca2+ sensitization in smooth muscle is initiated by activation of PKC and ROK (Somlyo and Somlyo, 2003). These enzymes phosphorylate various protein targets, resulting in inhibition of the MLCP and a consequent increase in phosphorylation of rMLC and contraction. Consistent with a role for PKC, we found that PMA-induced Ca2+ sensitization was prevented or reversed by the PKC inhibitor GF-109203X. We also found that thrombin-induced Ca2+ sensitization was inhibited by GF-109203X, suggesting that thrombin activates the PKC pathway in SMCs of the small airways. This notion is supported by a recent study showing that proteinase-activated receptor 1 stimulation with thrombin results in PKC activation, CPI-17 phosphorylation, inhibition of MLCP, and rMLC phosphorylation during assembly and contraction of stress fibers in retinal pigment epithelium (Ruiz-Loredo et al., 2012).
The phosphoprotein CPI-17, mainly expressed in smooth muscle, is a target for PKC that, when phosphorylated, binds to the PP1 subunit of MLCP to cause its inhibition, thereby causing Ca2+ sensitization (Wright et al., 2012). Here, we found that PKC activation induces ∼60% phosphorylation of CPI-17 in the airways of “normal” and “Ca2+-permeabilized” lung slices, suggesting that CPI-17 is a mediator of PMA-induced Ca2+ sensitization in small airways. CPI-17 phosphorylation was rapidly reversed by GF-109203X, indicating that phosphorylation of CPI-17 depends on continued PKC activation. Our results also suggest that this signal is quickly turned off upon termination of a stimulus, perhaps through constitutive phosphatase activity. Finally, we found that PKC activation caused a reversible increase in rMLC phosphorylation, as expected if phosphorylated CPI-17 inhibits MLCP. Thus, our data support a mechanism in which CPI-17 relays the signal from PKC in the intracellular cascade that increases rMLC phosphorylation and Ca2+ sensitization in small airway SMCs.
In conclusion, we suggest that activation of PKC in small airways promotes Ca2+ influx into SMC via LVGCs and, subsequently, intracellular Ca2+ release via RyR to generate low frequency Ca2+ oscillations and SMC twitching. PKC activation also induces a strong Ca2+ sensitization mediated by CPI-17 and rMLC phosphorylation. Finally, PKC activation by specific molecules, such as thrombin, that are present in the airways in conjunction with inflammatory lung diseases, could conceivably sensitize the airway SMCs to local agonists and contribute to airway hyperresponsiveness.
Supplementary Material
Acknowledgments
We are grateful to Dr. Luis Reuss for his critical reading of the manuscript and valuable suggestions.
This work was supported by grants from the American Heart Association (11SDG5670050), the American Lung Association (RG-196192-N), and a seed grant from the Center for Membrane Protein Research (CMPR) at Texas Tech University Health Sciences Center.
Richard L. Moss served as editor.
Footnotes
Abbreviations used in this paper:
- 5-HT
- serotonin
- ACh
- acetylcholine
- [Ca2+]i
- intracellular Ca2+ concentration
- CPA
- cyclopiazonic acid
- LVGC
- L-type voltage-gated Ca2+ channel
- MLCP
- myosin light chain phosphatase
- PDBu
- phorbol 12,13-dibutyrate
- rMLC
- regulatory myosin light chain
- ROI
- region(s) of interest
- ROK
- RhoA kinase
- SMC
- smooth muscle cell
References
- Bai Y., Sanderson M.J. 2006a. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir. Res. 7:34 10.1186/1465-9921-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Sanderson M.J. 2006b. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L208–L221 10.1152/ajplung.00494.2005 [DOI] [PubMed] [Google Scholar]
- Bai Y., Sanderson M.J. 2009. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 296:L947–L958 10.1152/ajplung.90288.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Edelmann M., Sanderson M.J. 2009. The contribution of inositol 1,4,5-trisphosphate and ryanodine receptors to agonist-induced Ca(2+) signaling of airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L347–L361 10.1152/ajplung.90559.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner A., Sanderson M.J. 2002. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J. Gen. Physiol. 119:187–198 10.1085/jgp.119.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel P.R. 2011. The role of small airways in obstructive airway diseases. Eur Respir Rev. 20:023–033 10.1183/09059180.00010410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J.D., Majoor C.J., van ’t Veer C., Bel E.H., van der Poll T. 2012. Asthma and coagulation. Blood. 119:3236–3244 10.1182/blood-2011-11-391532 [DOI] [PubMed] [Google Scholar]
- Delmotte P., Sanderson M.J. 2008. Effects of albuterol isomers on the contraction and Ca2+ signaling of small airways in mouse lung slices. Am. J. Respir. Cell Mol. Biol. 38:524–531 10.1165/rcmb.2007-0214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Sanderson M.J. 2010. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 42:373–381 10.1165/rcmb.2008-0403OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Ressmeyer A.R., Bai Y., Sanderson M.J. 2010. Mechanisms of airway smooth muscle relaxation induced by beta2-adrenergic agonists. Front. Biosci. 15:750–764 10.2741/3644 [DOI] [PubMed] [Google Scholar]
- Gabazza E.C., Taguchi O., Tamaki S., Takeya H., Kobayashi H., Yasui H., Kobayashi T., Hataji O., Urano H., Zhou H., et al. 1999. Thrombin in the airways of asthmatic patients. Lung. 177:253–262 10.1007/PL00007645 [DOI] [PubMed] [Google Scholar]
- Hauck R.W., Schulz C., Schömig A., Hoffman R.K., Panettieri R.A., Jr 1999. α-Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am. J. Physiol. 277:L22–L29 [DOI] [PubMed] [Google Scholar]
- Huang C.K., Munakata M., Baraban J.M., Menkes H. 1987. Protein kinase C and tracheal contraction at low temperature. J. Pharmacol. Exp. Ther. 243:270–280 [PubMed] [Google Scholar]
- Kanazawa H., Yoshikawa T. 2007. Up-regulation of thrombin activity induced by vascular endothelial growth factor in asthmatic airways. Chest. 132:1169–1174 10.1378/chest.07-0945 [DOI] [PubMed] [Google Scholar]
- Maki J., Hirano M., Hoka S., Kanaide H., Hirano K. 2010. Thrombin activation of proteinase-activated receptor 1 potentiates the myofilament Ca2+ sensitivity and induces vasoconstriction in porcine pulmonary arteries. Br. J. Pharmacol. 159:919–927 10.1111/j.1476-5381.2009.00591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough J.E., Yuan R., Suzuki M., Seyednejad N., Elliott W.M., Sanchez P.G., Wright A.C., Gefter W.B., Litzky L., Coxson H.O., et al. 2011. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 365:1567–1575 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C., Fortin S., Rousseau E. 2012. Bronchial inflammation induced PKCζ over-expression: involvement in mechanical properties of airway smooth muscle. Can. J. Physiol. Pharmacol. 90:261–269 10.1139/y11-117 [DOI] [PubMed] [Google Scholar]
- Morrison K.J., Vanhoutte P.M. 1991. Inhibition of airway smooth muscle tone by a phorbol ester in the guinea pig trachea: role of epithelium and receptor reserve of the contractile agent. J. Pharmacol. Exp. Ther. 259:198–204 [PubMed] [Google Scholar]
- Navedo M.F., Amberg G.C., Votaw V.S., Santana L.F. 2005. Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA. 102:11112–11117 10.1073/pnas.0500360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo M.F., Amberg G.C., Nieves M., Molkentin J.D., Santana L.F. 2006. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J. Gen. Physiol. 127:611–622 10.1085/jgp.200609519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper U., Knipp S.C., Thies B., Henke R. 1996. Activation of protein kinase C accelerates contraction kinetics of airway smooth muscle. Pflugers Arch. 432:R47–R52 [PubMed] [Google Scholar]
- Perez J.F., Sanderson M.J. 2005. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J. Gen. Physiol. 125:535–553 10.1085/jgp.200409216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zoghbi J.F., Sanderson M.J. 2007. Endothelin-induced contraction of bronchiole and pulmonary arteriole smooth muscle cells is regulated by intracellular Ca2+ oscillations and Ca2+ sensitization. Am. J. Physiol. Lung Cell. Mol. Physiol. 293:L1000–L1011 10.1152/ajplung.00184.2007 [DOI] [PubMed] [Google Scholar]
- Perez-Zoghbi J.F., Karner C., Ito S., Shepherd M., Alrashdan Y., Sanderson M.J. 2009. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm. Pharmacol. Ther. 22:388–397 10.1016/j.pupt.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zoghbi J.F., Bai Y., Sanderson M.J. 2010. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J. Gen. Physiol. 135:247–259 10.1085/jgp.200910365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Zhang J., Philipson K.D., Kotlikoff M.I., Blaustein M.P., Matteson D.R. 2010. Activation of L-type Ca2+ channels by protein kinase C is reduced in smooth muscle-specific Na+/Ca2+ exchanger knockout mice. Am. J. Physiol. Heart Circ. Physiol. 298:H1484–H1491 10.1152/ajpheart.00965.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressmeyer A.R., Bai Y., Delmotte P., Uy K.F., Thistlethwaite P., Fraire A., Sato O., Ikebe M., Sanderson M.J. 2010. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am. J. Respir. Cell Mol. Biol. 43:179–191 10.1165/rcmb.2009-0222OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti M., Savineau J.P., Crevel H., Marthan R. 1995. Role of protein kinase C in nonsensitized and passively sensitized human isolated bronchial smooth muscle. Am. J. Physiol. 268:L966–L971 [DOI] [PubMed] [Google Scholar]
- Ruiz-Loredo A.Y., López E., López-Colomé A.M. 2012. Thrombin stimulates stress fiber assembly in RPE cells by PKC/CPI-17-mediated MLCP inactivation. Exp. Eye Res. 96:13–23 10.1016/j.exer.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Sakai H., Yamamoto M., Kozutsumi Y., Chiba Y., Misawa M. 2009. Identification of PKC isoforms expressed in human bronchial smooth muscle cell. J. Smooth Muscle Res. 45:55–62 10.1540/jsmr.45.55 [DOI] [PubMed] [Google Scholar]
- Sakai H., Kurihara Y., Hashimoto Y., Chiba Y., Misawa M. 2010. Involvement of multiple PKC isoforms in phorbol 12,13-dibutyrate-induced contraction during high K(+) depolarization in bronchial smooth muscle of mice. J. Smooth Muscle Res. 46:225–233 10.1540/jsmr.46.225 [DOI] [PubMed] [Google Scholar]
- Sanderson M.J. 2011. Exploring lung physiology in health and disease with lung slices. Pulm. Pharmacol. Ther. 24:452–465 10.1016/j.pupt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson M.J., Parker I. 2003. Video-rate confocal microscopy. Methods Enzymol. 360:447–481 10.1016/S0076-6879(03)60123-0 [DOI] [PubMed] [Google Scholar]
- Sanderson M.J., Delmotte P., Bai Y., Perez-Zogbhi J.F. 2008. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc. Am. Thorac. Soc. 5:23–31 10.1513/pats.200704-050VS [DOI] [PubMed] [Google Scholar]
- Somlyo A.P., Somlyo A.V. 2003. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83:1325–1358 [DOI] [PubMed] [Google Scholar]
- Souhrada M., Souhrada J.F. 1989. Sodium and calcium influx induced by phorbol esters in airway smooth muscle cells. Am. Rev. Respir. Dis. 139:927–932 [DOI] [PubMed] [Google Scholar]
- Takeya K., Loutzenhiser K., Shiraishi M., Loutzenhiser R., Walsh M.P. 2008. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am. J. Physiol. Renal Physiol. 294:F1487–F1492 10.1152/ajprenal.00060.2008 [DOI] [PubMed] [Google Scholar]
- Wagers S.S., Norton R.J., Rinaldi L.M., Bates J.H., Sobel B.E., Irvin C.G. 2004. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J. Clin. Invest. 114:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.J., Tanna B. 2004. The interaction of ryanoids with individual ryanodine receptor channels. Biol. Res. 37:527–538 10.4067/S0716-97602004000400006 [DOI] [PubMed] [Google Scholar]
- Wright D.B., Tripathi S., Sikarwar A., Santosh K.T., Perez-Zoghbi J., Ojo O.O., Irechukwu N., Ward J.P., Schaafsma D. 2012. Regulation of GPCR-mediated smooth muscle contraction: Implications for asthma and pulmonary hypertension. Pulm. Pharmacol. Ther. In press [DOI] [PubMed] [Google Scholar]
- Yang K.X., Black J.L. 1995. The involvement of protein kinase C in the contraction of human airway smooth muscle. Eur. J. Pharmacol. 275:283–289 10.1016/0014-2999(94)00785-6 [DOI] [PubMed] [Google Scholar]
- Yang L., Liu G., Zakharov S.I., Morrow J.P., Rybin V.O., Steinberg S.F., Marx S.O. 2005. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J. Biol. Chem. 280:207–214 [DOI] [PubMed] [Google Scholar]
- Yang L., Doshi D., Morrow J., Katchman A., Chen X., Marx S.O. 2009. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c). Biochemistry. 48:6674–6683 10.1021/bi900322a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.