Abstract
Background
Nab-paclitaxel is a solvent-free, taxane-based chemotherapy approved for the treatment of metastatic breast cancer (mbc). This study reports clinical benefit and toxicities experienced by women with mbc treated with nab-paclitaxel at the Ottawa Hospital Cancer Centre.
Methods
Women with mbc treated with single-agent nab-paclitaxel between June 2006 and December 2010 were included in this analysis. Retrospective data obtained included demographics, disease characteristics, prior chemotherapy, nab-paclitaxel treatment, toxicity, and survival. Clinical benefit was defined as partial or complete response or stable disease (by clinical or radiologic evaluation, or both) at 6 months or more.
Results
Of 43 women (mean age: 57.0 years; range: 34–74 years), most had disease positive for estrogen or progesterone receptor (72.1%, 58.1%), or both. Nab-paclitaxel was administered weekly (qw: 44.2%), every 3 weeks (q3w: 46.5%), q3w switched to qw (7.0%), or qw switched to q3w (2.3%). Median duration of therapy was 5.1 months (qw) and 3.0 months (q3w). Sensory neuropathy was the primary toxicity (45.4% qw, 38.1% q3w; p = 0.62). Clinical benefit was observed in most women (76.2% qw, 57.1% q3w; p = 0.20). Women receiving nab-paclitaxel had a median overall survival of 13.6 months qw (range: 8.1–28.3 months) and 10.8 months q3w (range: 5.9–17.9 months; p = 0.03). Regardless of dosing schedule, women experiencing clinical benefit lived significantly longer than those not experiencing a benefit (17.3 months vs. 7.7 months; hazard ratio: 0.14; 95% confidence interval: 0.06 to 0.33).
Conclusions
Our clinical experience demonstrates that most women treated with nab-paclitaxel experienced some clinical benefit. Patients achieving clinical benefit lived significantly longer than those who did not. Nab-paclitaxel was well tolerated, with the primary toxicity being mild sensory neuropathy. Nab-paclitaxel represents another treatment option, with a favourable toxicity profile, for women with mbc.
Keywords: Nab-paclitaxel, taxane, mbc, Abraxane
1. INTRODUCTION
For women with metastatic breast cancer (mbc), taxanes1 are an established treatment option, both as monotherapy and in combination2,3. Paclitaxel and docetaxel are both hydrophobic, and to enable intravenous administration, they are formulated using the synthetic solvents polyoxyethylated castor oil (Cremophor EL: Sigma–Aldrich Canada, Oakville, ON) and polysorbate 80 (Tween 80: Sigma–Aldrich Canada)4. These synthetic solvents are associated with the development of acute hypersensitivity reactions and peripheral neuropathy4 and so require premedication with corticosteroids, antihistamines, and H2 a ntagonists (Taxotere P I: S anofi–Aventis, Bridgewater, NJ, U.S.A.; Taxol PI: Bristol–Myers Squibb, New York, NY, U.S.A.).
Nab-paclitaxel is a colloidal suspension of paclitaxel and human serum albumin that can be administered without premedication (Abraxane PI, Abraxane PM: Celgene Corporation, Summit, NJ, U.S.A.). The clinical efficacy of nab-paclitaxel [260 mg/m2 every 3 weeks (q3w)] compared with that of paclitaxel (175 mg/m2 q3w) was established in a randomized open-label phase iii study of 460 patients with mbc5. Most patients in that study had more than three metastatic lesions (76%), a high burden of visceral disease (79%), prior chemotherapy (86%), and progression after first-line therapy (59%). A significant improvement in the overall response rate (33% vs. 19%, p = 0.001) and a longer median time to progression (23.0 weeks vs. 16.9 weeks, p = 0.006) were observed with nab-paclitaxel compared with paclitaxel. The overall study population also showed a trend toward improved median overall survival5. Treatment with nab-paclitaxel was well tolerated. Despite a 49% higher paclitaxel dose, the incidence of grade 4 neutropenia was lower with nab-paclitaxel than with solvent-based paclitaxel (9% vs. 22%, p < 0.001), and although the incidence of sensory neuropathy was higher for nab-paclitaxel, that symptom improved rapidly (median: 22 days vs. 79 days for solvent-based paclitaxel)5,a.
The optimal dose and schedule of nab-paclitaxel has yet to be clearly defined. The results of a randomized phase ii study in women with mbc suggest that weekly dosing of nab-paclitaxel (qw: 100 mg/m2, 150 mg/m2) is feasible and may offer clinical benefit compared with q3w nab-paclitaxel (300 mg/m2) and docetaxel (100 mg/m2)6. Although grade 3 sensory neuropathy was observed more frequently with nab-paclitaxel (300 mg/m2 q3w, 2%; 100 mg/m2 qw, 9%; 150 mg/m2 qw, 22%), the time to onset was significantly longer than that reported in trials with paclitaxel (151–189 days depending on schedule vs. within 1–3 days and peaking around 27 days)7–9. As in the pivotal phase iii trial, nab-paclitaxel–related sensory neuropathy improved rapidly (≤grade 2: 20–22 days), thus potentially permitting resumption of systemic therapy. In addition, although persistent (≥2 years) sensory neuropathy has been reported in cross-sectional studies with paclitaxel, such long-term effects have not been observed to date in studies with nab-paclitaxel10. Recent evidence also suggests that, compared with q3w docetaxel, qw nab-paclitaxel (150 mg/m2) may confer a survival advantage in women with mbc (33.8 months vs. 26.6 months, p = 0.047)11.
In August 2006, nab-paclitaxel received approval for funding in Ontario for women with mbc. Eligible patients include those with acute infusion reactions with paclitaxel or docetaxel considered by treating physicians to be a result of the vehicle for the taxanes (Cremophor and polysorbate 80), severe toxicity from previous administration of other taxanes, or severe toxicity that might be attributable to premedications (for example, steroids) used for the administration of the taxane. Here, we report our “real world” clinical experience with the use of nab-paclitaxel in women with mbc at a single academic cancer centre in Ontario.
2. METHODS
This analysis includes mbc patients treated with single-agent nab-paclitaxel at the Ottawa Hospital Cancer Centre between June 2006 and December 2010. The data collected included age, date of diagnosis, surgery, estrogen and progesterone receptor status, human epidermal growth factor receptor 2 status, sites of metastasis, initial tumour stage and lymph node status, histologic diagnosis, endocrine therapy, prior chemotherapy regimens, nab-paclitaxel administration (duration of therapy, dosing schedule, reasons for nab-paclitaxel use), toxicities, dose reductions, clinical benefit, and date of death. Toxicities were graded using the Common Terminology Criteria for Adverse Events, version 4.0, based on the description provided in the clinical progress notes12.
The study was approved by the Ottawa Hospital Research Ethics Board.
2.1. Eligibility Criteria
Eligible patients for this study included all women with mbc who were treated with single-agent nab-paclitaxel at the Ottawa Hospital Cancer Centre. Patients who had received taxane (docetaxel or paclitaxel) chemotherapy (adjuvant or metastatic setting) before receiving nab-paclitaxel were also included. Patients who received coverage for nab-paclitaxel through the Ontario New Drug Funding Program or third-party funding (private insurance) were included.
2.2. Therapy
Patients received nab-paclitaxel (260 mg/m2) intravenously over 30 minutes q3w or 100 mg/m2 intravenously over 30 minutes qw for 3 weeks of every 4 at the discretion of the treating oncologist. Steroid premedication was not required before administration of therapy.
2.3. Clinical Benefit
Radiologic and clinical assessments were performed at the discretion of the treating physician. Clinical benefit was measured using the Response Evaluation Criteria in Solid Tumors13 and was defined as complete response (no clinical or radiologic evidence of disease), partial response (decrease of at least 30% in the sum of lesions), or stable disease (clinically or radiologically, ≤30% decrease or ≤20% increase in lesions for a duration of ≥6 months).
2.4. Statistical Analysis
Demographic and clinical data are summarized descriptively as means, medians, or proportions. Survival curves were generated using the Kaplan–Meier method and were compared using the log-rank test. Patients alive or lost to follow up as of December 31, 2010, were censored. In an exploratory analysis, the relative risk of death for various patient subgroups was estimated using Cox proportional hazards regression. All of the statistical analyses were performed using the Stata software package (release 11.0: Stata Corp., College Station, TX, U.S.A.).
3. RESULTS
3.1. Patients
Between June 2006 and December 2010, 43 patients with mbc received treatment with single-agent nab-paclitaxel at the Ottawa Hospital Cancer Centre, and 42 patients were evaluable for clinical response. Table i summarizes patient demographics and treatment characteristics. In this group, mean age was 57 years (range: 34–74 years), and most patients (72.1%) had estrogen receptor–positive disease. Most patients also had several sites of metastases (81.5%) and had received multiple lines of chemotherapy (median = 3; range: 1–6) before receiving nab-paclitaxel.
TABLE I.
Demographic and treatment characteristics of patients with metastatic breast cancer receiving nab-paclitaxel
| Variable | Value |
|---|---|
| Patients (n) | 43 |
| Age (years) | |
| Mean | 57.0 |
| Range | 34–74 |
| Receptor status (%) | |
| er-positive | 72.1 |
| pr-positive | 58.1 |
| her2-positive | 16.3 |
| Sites of metastasis (%) | |
| Bone only | 11.6 |
| Liver only | 2.3 |
| Lung only | 4.6 |
| Multiple sites | 81.5 |
| Current line of chemotherapy for advanced disease [median (range)] | 3 (1–6) |
| Previous taxane exposure [n (%)] | 31 (72.1) |
| Adjuvant | 6 (19.35) |
| Metastatic | 23 (74.2) |
| Adjuvant and metastatic | 2 (6.45) |
| Nab-paclitaxel treatment | |
| Schedule [n (%)] | |
| Weekly (qw) | 19 (44.2) |
| Every 3 weeks (q3w) | 20 (46.5) |
| q3w, then switch to qw | 3 (7.0) |
| qw then switch to q3w | 1 (2.3) |
| Mean starting dose (mg) | |
| qw | 222±99.6 |
| q3w | 404±110 |
| Dose reductions (%) | |
| qw | 27.3 |
| q3w | 61.9 |
| Cycles [median (range)] | |
| qw | 12 (1–36) |
| q3w | 5 (2–22) |
| Median duration (months) | |
| qw | 5.1 |
| q3w | 3.0 |
| os for the entire cohort (months) | |
| Median | 11.9 |
| Interquartile range | 7.7–21.8 |
er = estrogen receptor; pr = progesterone receptor; her2 = human epidermal growth factor receptor 2; os = overall survival.
3.2. Nab-Paclitaxel Therapy
Nab-paclitaxel treatment was given to 20 patients (46.5%) in a q3w schedule for a median duration of 3.0 months, and to 19 patients (44.2%) in a qw schedule for a median duration of 5.1 months. Treatment was initially given in a qw schedule and switched to a q3w schedule in 1 patient (2.3%), and initially given in a q3w schedule and switched to a qw schedule in 3 patients (7.0%). More than twice as many dose reductions occurred in the nab-paclitaxel q3w group (61.9%) as in the qw group (27.3%). Reasons for dose reductions included neuropathy (n = 6), decreased body surface area (n = 2), feeling unwell (n = 2), switch of treatment schedule (n = 3), mucositis (n = 1), decreased Eastern Cooperative Oncology Group performance status (n = 1), wound infection (n = 1), fatigue (n = 1), neutropenia (n = 1), and other (n = 1). Most patients (n = 31, 72.1%) had already been exposed to taxanes in the adjuvant (19.4%), metastatic (74.2%), or adjuvant and metastatic (6.5%) settings.
3.3. Toxicity
Peripheral sensory neuropathy was the most commonly reported toxicity, being reported in approximately 41.9% (n = 18) of all women treated with nab-paclitaxel (45.4% qw vs. 38.1% q3w, Table ii). Five patients (11.6%) experienced significant (grade 3) peripheral sensory neuropathy. Grade 2 fatigue was reported in 4 patients (9.3%), and grade 3 myalgia, dyspnea, mucositis were each reported in 1 patient (2.3%). One patient experienced febrile neutropenia.
TABLE II.
Clinical outcomes data in patients with metastatic breast cancer receiving nab-paclitaxel on a weekly (qw) and every-3-weeks (q3w) schedule
| Variable |
Nab-paclitaxel schedule
|
p Value | |
|---|---|---|---|
| qw (100 mg/m2) | q3w (260 mg/m2) | ||
| Patients (n) | 21 | 21 | |
| Clinical benefit achieved (%)a | 76.2 | 57.1 | 0.20 |
| Development of neuropathy (%) | 45.4 | 38.1 | 0.62b |
| Overall survival (months) | |||
| Median | 13.6 | 10.8 | 0.033c |
| Interquartile range | 8.1–28.3 | 5.9–17.9 | |
Odds ratio for achieving clinical benefit with the weekly schedule was 2.5 (95% confidence interval: 0.64 to 9.0).
By chi-square test.
By log-rank test.
3.4. Clinical Benefit and Overall Survival
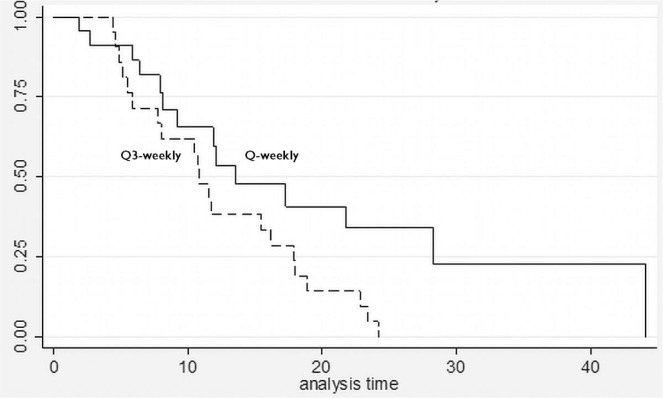
Clinical benefit was evaluable in 42 patients. Most women receiving nab-paclitaxel qw (76.2%) and just more than half the women on the q3w schedule (57.1%) experienced clinical benefit (Table ii). The odds ratio for achieving clinical benefit with the qw schedule was 2.5 relative to the q3w schedule [95% confidence interval (ci): 0.64 to 9.0; p = 0.20], suggesting that qw should be the preferred schedule for drug delivery. A partial response to nab-paclitaxel therapy was seen in 4 patients (9.5%: 3 qw, 1 q3w), and a best response of stable disease was seen in 25 patients (59.5%: 14 qw, 11 q3w). Women who received nab-paclitaxel qw had a median overall survival of 13.6 months compared with 10.8 months in the q3w group (Figure 1).
FIGURE 1.
Survival curves for patients receiving weekly (Q-weekly) and every-3-weeks (Q3-weekly) nab-paclitaxel. Median survivals were 13.6 months for nab-paclitaxel Q-weekly and 10.8 months for nab-paclitaxel Q3-weekly (adjusted log-rank p = 0.03; hazard ratio: 0.47; 95% confidence interval: 0.23 to 0.96).
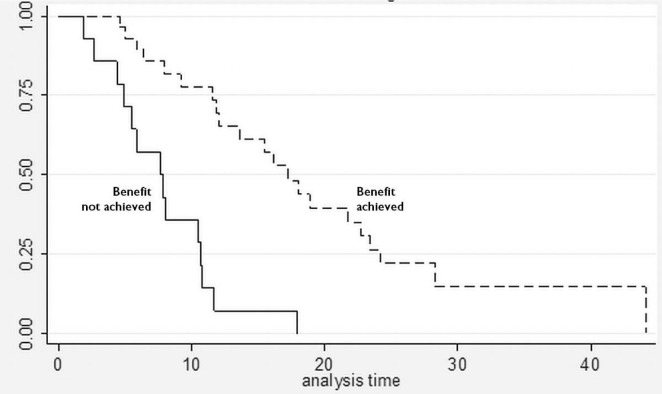
Regardless of the schedule of administration, women who experienced clinical benefit from nab-paclitaxel lived significantly longer than those who did not achieve clinical benefit [17.3 months vs. 7.7 months; hazard ratio (hr): 0.14; 95% ci: 0.06 to 0.33; p < 0.001; Figure 2]. In the Cox proportional hazards analysis, achievement of clinical benefit (hr: 0.14; p = 0.001) was a predictor of improvement in overall survival, and exposure to multiple lines of chemotherapy (hr: 1.39; p = 0.021) was associated with a decrease in overall survival (Table iii). Clinical benefit was achieved in 24 patients (77.4%) who had prior exposure to taxanes (n = 31). Of the 42 evaluable patients, 13 (31%) progressed while on nab-paclitaxel treatment.
FIGURE 2.
Survival curves for patients who achieved and did not achieve clinical benefit from nab-paclitaxel. Median survivals were 7.7 months for patients not achieving clinical benefit and 17.3 months for patients achieving clinical benefit (adjusted log-rank p < 0.001; hazard ratio: 0.14; 95% confidence interval: 0.06 to 0.33).
TABLE III.
Results of Cox proportional hazards analysis on overall survivala
| Variable | hr | 95% ci | p Value | Impact on survival |
|---|---|---|---|---|
| Line of chemotherapy | 1.39 | 1.05 to 1.84 | 0.021 | ↑ Risk by 39% per line |
| Achievement of clinical benefita | 0.14 | 0.06 to 0.33 | 0.001 | ↓ Risk by 86% |
Schedule of nab-paclitaxel was not a significant predictor of overall survival, nor was the interaction between nab-paclitaxel schedule and achievement of clinical benefit.
4. DISCUSSION
Traditional solvent-based taxanes have been shown to be beneficial in the treatment of mbc. The “down-side” to this class of drugs has been related mainly to the inherent difficulties of drug delivery: the need for solvent, special tubing, and premedication with dexamethasone to prevent allergic reactions. In addition, it has been suggested that the Cremophor solvent used in the preparation of paclitaxel may have a direct negative effect on the antitumour properties of that drug14. Paclitaxel is entrapped by the formation of plasma Cremophor EL micelles, which can cause reduced drug clearance, nonlinear pharmacokinetics, and free drug fraction14. The dose-dependent antitumour response from paclitaxel can be decreased as a result15. It is this drug entrapment phenomenon that partly explains why giving higher doses of solvent-based paclitaxel does not result in improved clinical efficacy16.
Nab-paclitaxel is a novel formulation of albumin-bound paclitaxel that has been shown in clinical trials to provide clinical benefit without the need for solvents and with an acceptable toxicity profile. Importantly, nab-paclitaxel has demonstrated limited taxane cross-resistance. That feature is of particular clinical importance for women with early-stage breast cancer who relapse within 12 months of exposure to a solvent-based taxane. A phase ii study17 of weekly nab-paclitaxel monotherapy in women with taxane-refractory mbc reported 13 partial responses (20%) among 66 evaluable patients, with 7 patients responding and 3 having stable disease (for at least 24 weeks). Those observations suggest that nab-paclitaxel may provide long-term disease control in the difficult-to-treat taxane-refractory mbc population. The issue of lack of cross-resistance is being evaluated more rigorously in the ongoing multicentre single-arm phase ii tiffany trial (search for NCT01416558 at http://clinicaltrials.gov) sponsored by the German Breast Group.
In our small single-institution retrospective study, most women with mbc who received nab-paclitaxel experienced some degree of clinical benefit (qw 76.2% vs. q3w 57.1%), with the greatest proportion of clinical benefit being observed as stable disease. Notably, clinical benefit was also seen in heavily pretreated women (median of 3 cycles of chemotherapy before nab-paclitaxel) and those previously exposed to taxanes in the adjuvant and metastatic settings. Women who experienced clinical benefit from nab-paclitaxel survived significantly longer than those who did not (17.3 months vs. 7.7 months, p < 0.001). Nab-paclitaxel was well tolerated, with mild sensory neuropathy (grade 1 or 2) occurring in approximately 27.9% of patients. Dose reductions, mainly because of toxicity, were more common with q3w administration (61.9%) than with qw administration (27.3%), implying that the latter schedule of administration was better tolerated.
The results of a randomized phase iii trial [Cancer and Leukemia Group B (calgb) 40502/North Central Cancer Treatment Group (ncctg) N063H] of nab-paclitaxel or ixabepilone or paclitaxel with or without bevacizumab in women with chemotherapy-naïve mbc, was recently presented. The second interim analysis demonstrated that nab-paclitaxel was highly unlikely to be superior to paclitaxel for progression-free survival (9.2 months vs. 10.6 months; hr: 1.19; 95% ci: 0.96 to 1.49)18. Patients receiving paclitaxel experienced a lower incidence of sensory neuropathy (37% vs. 48%) and fewer hematologic toxicities (12% vs. 49%). Those results differ from the findings reported from the phase iii trial by Gradishar et al.5, which demonstrated a superior overall response rate (33% vs. 19%, p = 0.001) and overall survival (56.4 weeks vs. 46.7 weeks; hr: 0.73; p = 0.024) in the second-line or greater setting with nab-paclitaxel than with paclitaxel. These differences in trial results may reflect different patient populations (chemotherapy-naïve vs. pretreated), use of bevacizumab, and varying doses and schedules of taxanes (nab-paclitaxel vs. Taxol).
The role of sparc [secreted protein, acidic and rich in cysteine, a form of the caveolin 1 (CAV) gene] as a biomarker for nab-paclitaxel effectiveness is being explored further in the calgb 40502/ncctg N063H trial (search for NCT00785291 at http://clinicaltrials.gov). That trial will evaluate the relationships of sparc overexpression and of changes in blood levels of caveolin 1 with progression-free survival and secondary endpoints of response during treatment.
Several limitations in the present study need to be acknowledged. The small study population and retrospective nature of the analysis represent significant limitations in interpreting its results. Data were collected retrospectively on a select group of patients who received nab-paclitaxel if they had private insurance coverage or met the eligibility criteria for the Ontario New Drug Funding Program, creating a potential for selection bias. Clinical benefit was based on clinical and radiologic assessments (for example, computed tomography imaging) of patients, which were requested by the treating physicians at variable points in time. In addition, there was a potential imbalance in prognostic factors among patients who received the qw schedule and those who received the q3w schedule of nab-paclitaxel.
5. CONCLUSIONS
In our experience, most women with mbc treated with single-agent nab-paclitaxel (some having received up to 6 prior lines of chemotherapy) experienced some degree of clinical benefit with an acceptable level of toxicity. Although the median overall survival for all women treated with nab-paclitaxel was 11.9 months, patients who experienced a clinical benefit lived longer (overall survival: 17.3 months). Our “real world” experience suggests that nab-paclitaxel can safely be offered to women with mbc, with reasonable expectations of clinical benefit and without concern of significant toxicity. The divergent results and comparative limitations of the second interim analysis of the calgb 40502/ncctg N063H phase iii trial presented at the American Society of Clinical Oncology 2012 annual meeting do not negate the potential benefit of nab-paclitaxel in the treatment of women with mbc in second-line treatment or beyond. In Ontario, given the current Cancer Care Ontario funding model, the results of the calgb 40502/ncctg NO63H study are unlikely to have significant impact on the utilization of nab-paclitaxel in women with mbc.
Footnotes
Abraxis BioScience, data on file.
6. CONFLICT OF INTEREST DISCLOSURES
This study was supported with an unrestricted educational grant provided by Celgene Canada (formerly Abraxis BioSciences). SD has received research support from Celgene Canada, but no compensation was received for the preparation of the present manuscript. The remaining authors have no financial conflicts of interest to disclose.
7. REFERENCES
- 1.Aapro MS, Minckwitz GV. Molecular basis for the development of novel taxanes in the treatment of metastatic breast cancer. EJC Supplements. 2008;6:3–11. [Google Scholar]
- 2.National Comprehensive Cancer Network (nccn) Breast Cancer. Fort Washington, PA: NCCN; 2010. NCCN Clinical Practice Guidelines in Oncology Ver 1.2010. [Google Scholar]
- 3.Cardoso F, Castiglione M, on behalf of the esmo Guidelines Working Group Locally recurrent or metastatic breast cancer: esmo Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):15–18. doi: 10.1093/annonc/mdp115. [DOI] [PubMed] [Google Scholar]
- 4.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase iii trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 6.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–19. doi: 10.1200/JCO.2008.18.5397. [Erratum in: J Clin Oncol 2011;29:2739] [DOI] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Krasnojon D, Cheporov SV, et al. Albumin-bound paclitaxel (ab-pac) versus docetaxel for first-line treatment of metastatic breast cancer (mbc): final overall survival (os) analysis of a randomized phase ii trial [abstract 275] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=111&abstractID=86392; cited November 26, 2012] [Google Scholar]
- 8.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–57. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4:165–72. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–74. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, Krasnojon D, Cheporov S, et al. Phase ii trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer. 2012;12:313–21. doi: 10.1016/j.clbc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 12.United States, National Institutes of Health, National Cancer Institute (nci) Common Terminology Criteria for Adverse Events (CTCAE) Bethesda, MD: NCI; 2009. Ver. 4.0. [Available online at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf; cited June 1, 2010] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor EL–mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59:1454–7. [PubMed] [Google Scholar]
- 15.Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–5. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B trial 9342. J Clin Oncol. 2004;22:2061–8. doi: 10.1200/JCO.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Blum JL, Savin MA, Edelman G, et al. Long term disease control in taxane-refractory metastatic breast cancer treated with nab paclitaxel [abstract 543] J Clin Oncol. 2004;22 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=26&abstractID=3975; cited November 26, 2012] [Google Scholar]
- 18.Rugo HS, Barry WT, Moreno–Aspitia A, et al. calgb 40502/ncctg N063H: randomized phase iii trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nab-paclitaxel or ixabepilone with or without bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer [abstract CRA1002] J Clin Oncol. 2012;30 doi: 10.1200/JCO.2014.59.5298. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=99475; cited November 26, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai NP, Trieu V, Hwang LY, Wu R, Soon–Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of her2 and sparc status. Anticancer Drugs. 2008;19:899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]