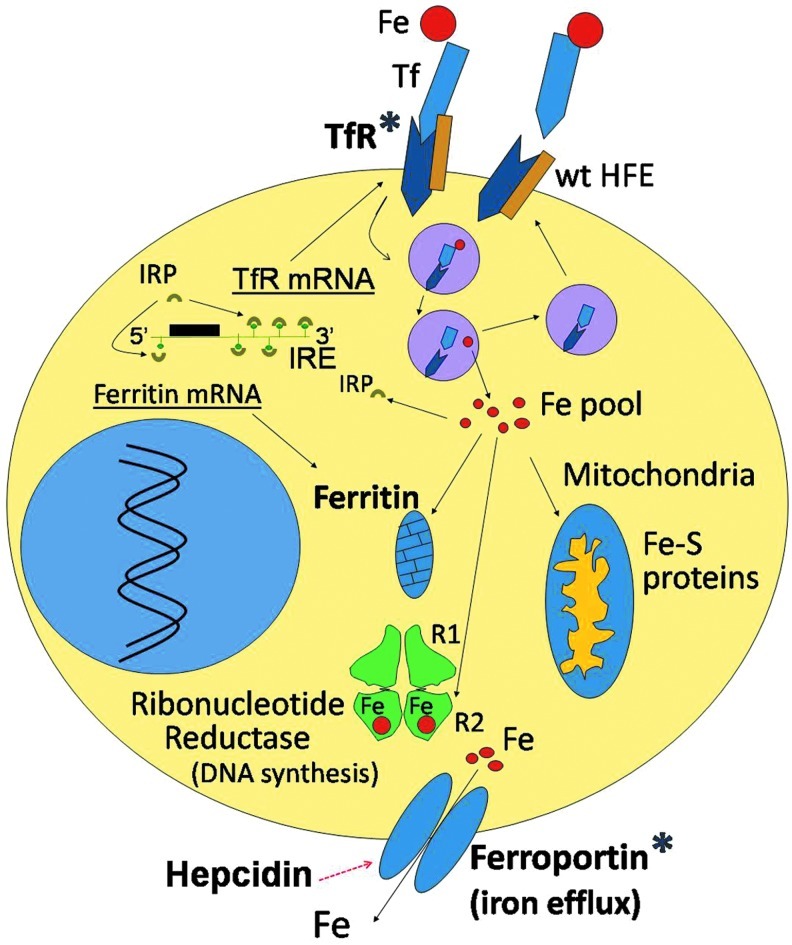
FIG. 1.
Iron proteins in breast cancer cells. Under physiologic conditions, iron is bound to transferrin (Tf) in the circulation and is incorporated into cells by transferrin receptor1 (TfR1)-mediated endocytosis of Tf-Fe complexes. The binding site of the wild-type hemochromatosis protein (wt HFE) partially overlaps with the Tf-binding site on TfR1 and can, thus, competitively inhibit Tf binding to its receptor. This regulatory effect of HFE on Tf-Fe-TfR binding is lost with the HFE C282Y mutation, as the latter is degraded within the cell and no longer associates with the TfR to interfere with its binding to Tf-Fe. The Tf-Fe-TfR complex translocates from the cell surface to an intracellular acidic endosome, where Fe(III) dissociates from Tf and is reduced to Fe(II) by STEAP3 (six-membrane epithelial antigen of the prostate 3) (not shown). Fe(II) exits the endosome through divalent metal transporter1 (DMT1, not shown) to a labile iron “pool.” From here, iron trafficks to different compartments (mitochondria, ribonucleotide reductase [RR], and others). Excess iron is stored in ferritin. Iron exits from the cell through cell membrane-based ferroportin. Ferroportin levels can be lowered by hepcidin, which binds to it and translocates it to the lysosome for degradation. Cytoplasmic iron regulatory proteins (IRPs) function as sensors of cellular iron status and regulate the synthesis of Tf receptors, ferritin, and ferroportin at the mRNA translational level by interactions with iron response elements (IREs) present in the untranslated regions of their respective mRNAs. Iron proteins known to be altered in breast cancer are marked with an asterisk (*) and include an increase in TfR and ferritin as well as a reduction in ferroportin levels. In addition, the C282Y HFE mutation may be associated with an increased risk of breast cancer development. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)