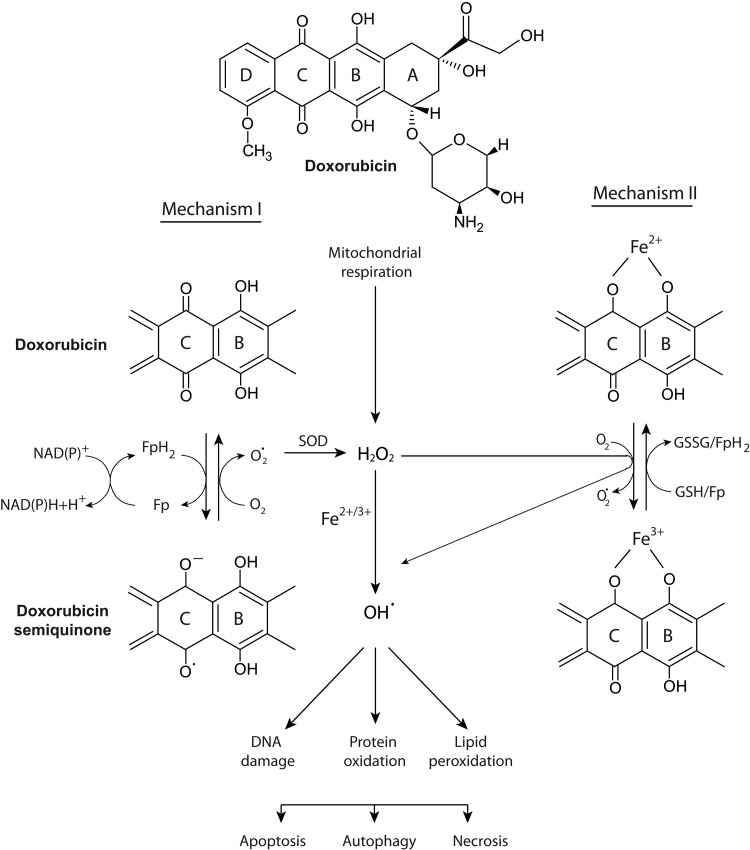
FIG. 1.
Currently understood mechanism for the iron-mediated generation of ROS by doxorubicin. The anthracycline doxorubicin undergoes a one-electron reduction of the C ring, leading to the formation of a semiquinone free radical metabolite. In the presence of oxygen, its unpaired electron is donated to oxygen forming superoxide radicals. Flavoproteins and glutathione (GSH/GSSG) catalyze the formation of a reduced semiquinone by accepting electrons from NADH or NADPH. SOD can catalyze the dismutation of superoxide into oxygen and H2O2 and provide an antioxidant defense, along with catalase and other antioxidant enzymes. A detailed description of the Fenton and Haber–Weiss reactions and the pathways labeled as Mechanism I or II are provided in the text. The iron-mediated generation of hydroxyl radicals can damage lipids, proteins, and DNA. The outcome of oxidative damage on critical cellular components could include apoptosis, autophagy, and/or necrosis. Modified with permission from Thomas Simunek (Charles University in Prague) (237). GSH, glutathione; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; NADH, nicotinamide adenine dinucleotide; ROS, reactive oxygen species; SOD, superoxide dismutase.