Abstract
Background
Troxacitabine is a novel L-nucleoside analogue. Preclinical studies showed improved activity with infusions of at least 3 days compared with bolus regimens, especially at concentrations >20 ng/ml. This phase I study tested the feasibility of achieving a troxacitabine steady-state concentration of 20 ng/ml for at least 72 h in patients with solid tumors.
Patients and methods
Patients with solid tumors received troxacitabine as a progressively longer infusion on days 1–4 of a 28-day cycle. The initial length of infusion and infusion rate were 48 h and 3 mg/m2/day.
Results
Twenty-one patients were treated at infusion lengths that increased from 48 to 72 h and then 96 h. The infusion rate was decreased from 3 to 1.88 mg/m2/day due to toxicity. Dose-limiting toxicities consisted of grade 4 neutropenia (three) and grade 3 constipation (one). The maximum tolerated dose of continuous infusion troxacitabine in patients with solid tumors is 7.5 mg/m2 administered over 96 h. This dose level resulted in steady-state drug concentration of at least 20 ng/ml for 72 h.
Conclusions
Administration of troxacitabine by continuous infusion achieved the prospectively defined target plasma concentration. Pharmacokinetics (PK) modeling coupled with real-time PK assessment was an efficient approach to conduct hypothesis-driven phase I trials.
Keywords: continuous infusion, PK modeling, troxacitabine
introduction
Troxacitabine (Troxatyl®; BCH-4556; SPD758; (–)-2-(S)-hydroxymethyl-4-(S)-(cytosin-1′-yl)-1,3-dioxolane; SGX Pharmaceuticals, San Diego, CA) is a nucleoside analogue different from gemcitabine and cytarabine in its structure, pharmacokinetics (PK), cellular transport, and mechanisms of resistance. The agent possesses potent in vivo antitumor activity in animal models and has been studied in patients. Phase I studies of troxacitabine using different bolus administration schedules (every 3 weeks and daily 5× monthly) have been reported in patients with solid tumors [1, 2]. Neutropenia and skin toxicity were dose limiting in these studies that did not show impressive clinical activity.
Preclinical data indicate that continuous i.v. infusion of troxacitabine may be superior to bolus administration. Troxacitabine slowly permeates cells by carrier-mediated diffusion; thus, sustained (72 h) troxacitabine exposure was significantly more cytotoxic than 1 h exposure using cells from freshly removed patient tumors [3]. For example, the troxacitabine concentration that causes 50% inhibition of growth in leukemic HL60 cells is 0.6 μM after 1 h and 0.015 μM after 72 h, which correspond to 135 and 3 ng/ml, respectively. Xenograft experiments further demonstrated that prolonged exposures to low micromolar concentrations of troxacitabine lead to significant inhibition of tumor growth without the need to achieve high-peak drug concentrations [4]. Also, tumor growth arrest was seen following prolonged exposures to troxacitabine that achieved plasma concentrations below 50 ng/ml in a HT-29 human colorectal xenograft model [5].
Subsequent phase I and phase I/II studies in acute leukemia supported these findings, with prolonged infusion studies showing evidence of increased activity compared with bolus administration [6–8]. In a phase I study of troxacitabine given as a 30-min i.v. infusion daily for 5 days in 30 patients with refractory acute leukemia, a maximum tolerated dose (MTD) of 8 mg/m2/d for 5 days and a complete response (CR) rate of 10% were documented (defined as normalization of the blood and bone marrow with 5% or less blasts, granulocyte count ≥1000/μl, and platelet count ≥100 000/μl) [6]. In a small subsequent phase II study of troxacitabine administered following this schedule, there were two CRs (12%) in 16 patients [7]. In a larger phase I/II study that treated 48 patients assessing a prolonged (2–5 days) continuous infusions of troxacitabine, the MTD was 12 mg/m2/day for 5 days and the CR rate was 15% (17% in patients who received cumulative doses of troxacitabine of 40.4 mg/m2 or higher). Mucositis and hand–foot syndrome (HFS) were dose limiting in both phase I studies. In addition, several solid tumor phase II clinical trials in renal cell [9], pancreatic [10], and non-small-cell lung cancer [11] using short infusions have rendered homogeneously negative efficacy results. The most commonly observed toxic effects in the phase II studies were hematological (neutropenia) and cutaneous (skin rash, dry skin, pruritus, and HFS).
On the basis of these clinical and preclinical data, a dose- and infusion length-finding study of troxacitabine was initiated to determine the feasibility of achieving a minimum steady-state plasma concentration of 20 ng/ml (0.1 μM) for at least 72 h in subjects with solid tumors. The starting infusion rate for the present study (3 mg/m2/day) was selected on the basis of PK simulation modeling using data obtained from prior phase I studies [12]. The objectives were to identify an optimal continuous i.v. infusion dosing schedule, characterize the PK, and make an initial assessment of troxacitabine efficacy in adult patients with solid cancers when given as a protracted infusion.
patients and methods
patient eligibility
Patients were required to have histologically confirmed malignancy that was metastatic or unresectable and for which standard curative or palliative measures did not exist, age ≥18, Eastern Coperative Oncology Group performance status of two or less, life expectancy of 12 weeks or longer, adequate bone marrow, hepatic, and renal function [absolute neutrophil count ≥ 1500/μl, platelet count ≥ 100 000/μl, hemoglobin ≥ 9 g/dl, bilirubin level ≤ 2.0/dl, aspartate aminotransferase (AST) or alanine transaminase (ALT) levels ≤ 3.0 × the upper limit of normal (ULN), AST or ALT levels ≤ 5.0 × the ULN if hepatic metastases existed, and creatinine level ≤ 1.5 × the ULN or estimated clearance (CL) ≥ 45 ml/min]. Patients could have received any prior chemotherapy or radiation therapy, as long as it was terminated 28 days before study initiation. Measurable disease was not a requirement for study entry. Patients with brain metastases were not allowed. Prior treatment with more than six courses of alkylating agent-containing chemotherapy (except low-dose cisplatin) or more than four courses of carboplatin, radiation therapy to >25% of hematopoietic reserves or two or more courses of mitomycin C or nitrosourea was not allowed. Patients who had not recovered from adverse events due to agents administered >4 weeks earlier, and those with severe, clinically significant and/or uncontrolled medical conditions were excluded. The institutional review board of our institution granted protocol approval. Patients were required to provide written informed consent before enrollment into the study.
study objectives
The primary objectives of the trial were (i) to determine the dose-limiting toxicities (DLTs) and MTD of troxacitabine administered as a continuous infusion in patients with solid tumor and (ii) to investigate the clinical pharmacology of troxacitabine when administered in this fashion in order to determine the safest troxacitabine infusion dose rate that resulted in a predefined targeted plasma concentration over the specified durations. Secondary objectives included assessing antitumor activity of troxacitabine. Adverse events were classified and graded according to the National Cancer Institute—Common Toxicity Criteria version 2.0. The criteria used to define response were standard Response Evaluation Criteria for Solid Tumors [13]. Briefly, a partial response is defined as at least 30% decrease in the sum of the longest diameter of lesions. Progressive disease (PD) is defined as a 20% increase in the sum of the LD of lesions or the appearance of new ones. Stable disease (SD) is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Patients were considered assessable for toxicity and efficacy if at least a cycle was administered.
treatment plan
Troxacitabine was supplied by SGX Pharmaceuticals. Troxacitabine was administered as a continuous infusion starting on day 1 of a 28-day cycle, in an outpatient setting, via a central catheter. The first group of subjects was infused for 48 h, and the infusion length escalation was done in increments of 24 up to 96 h. The starting infusion rate of troxacitabine was 3 mg/m2/day. There were no plans to escalate the infusion rate, as the priority was to increase delivered dose by a maximum of 50% by lengthening the infusion time. Total dose reductions of 33% and 25% were preplanned by reducing the infusion rate. No prophylactic antiemetics were administered. In the absence of treatment delays due to adverse events, treatment was administered until disease progression, intercurrent illness that prevented further administration of treatment, unacceptable adverse events, or withdrawal of consent. Hematology and chemistries were conducted weekly during the first two cycles and then every other week for cycles 3 and thereafter. Retreatment required adequate parameters on every evaluation as per the above description and resolution of all non-hematological toxic effects (except alopecia and fatigue) to baseline or less than grade 1. In case of a delay longer than 14 days, the patient was removed from the study.
assessments, follow-up, and monitoring
Toxic events were recorded on a continuous basis, and followed until they were resolved to baseline or less than grade 1. Immediately before study entry, patients had a complete clinical history and physical examination, performance status assessment, complete blood count/differential, platelet count, chemistry screen (including creatinine level, electrolytes, total bilirubin level, alkaline phosphatase, AST, ALT, protein, calcium, phosphate, albumin, and glucose), urinalysis, chest X-ray, electrocardiogram, and disease assessment by computed tomography (CT) scan. A pregnancy test (urine or serum) was carried out for women of childbearing potential. CT scan of disease sites was repeated every two cycles.
definition of DLT, MTD, and dose escalation plan
DLT was defined as grade 4 neutropenia lasting ≥7 days, grade 3 or greater thrombocytopenia, and any grade 3 or 4 major organ toxicity (including febrile neutropenia), except skin rash in subjects not receiving prophylactic prednisone, or alopecia, or nausea/vomiting in subjects who are not receiving optimal antiemetic premedication and/or treatment. Also, delay of the initiation of the subsequent course of chemotherapy by >2 weeks (>14 days following day 28 of the first cycle) due to toxicity was considered as DLT. Only DLT determinations in the first cycle of therapy were applied for dose escalation decisions. Three patients were treated at each dose level. If no DLTs were encountered in any of the three patients in cycle 1, infusion length escalation was allowed. If one of the three patients experienced a DLT, three more patients were to be enrolled at the same dose level and if none of these three additional patients experienced a DLT, infusion length escalation was allowed. The recommended infusion schedule is defined as the longest infusion length and highest dose of troxacitabine at which less than two of six subjects experience DLTs during cycle 1.
PK sampling and analytic assay
PK studies were done in all patients. Blood samples were taken in the 48-h infusion pretreatment, 4, 23.5, and 47.5 h after the start of infusion; in the 72-h infusion pretreatment, 4, 23.5, 47.5, and 71.5 h after the start of infusion; and in the 96-h infusion pretreatment, 4, 23.5, 47.5, 71.5, and 95.5 h after the start of infusion. At each time point, one 5 ml blood sample was collected in a heparinized tube and immediately placed on ice. Within 30 min of collection, plasma was isolated by centrifugation at 4°C, 1000 g for 10 min, two equal aliquots were transferred to two polypropylene freezer vials and then frozen at –70° C until analytical analysis. Total plasma concentrations of troxacitabine were determined by high-performance liquid chromatography with mass spectrometry detection (LC/MS/MS) [1, 2].
PK data analysis
The PK profile of troxacitabine administered as a continuous infusion was characterized and the steady-state plasma concentrations (Css) were estimated for individual patients from the observed concentrations measured during the infusion. CL was calculated as the infusion rate divided by Css.
results
patient characteristics
Twenty-one patients with advanced solid tumors were accrued into the study. All patients were assessable for toxicity and efficacy. Demographic and clinical characteristics of the subjects are summarized in Table 1. A total of 48 cycles of the study drug were delivered (median, two cycles; range, 1–7).
Table 1.
Patient characteristics
| Characteristic | Number of patients (n = 21) |
|---|---|
| Sex | |
| Male | 12 |
| Female | 9 |
| Age, years | |
| Median | 61 |
| Range | 43–77 |
| ECOG performance status | |
| 0 | 3 |
| 1 | 16 |
| 2 | 2 |
| Tumor type | |
| Pancreatic | 6 |
| Colorectal | 4 |
| Renal cell | 2 |
| Adenocarcinoma, unknown primary | 2 |
| Appendiceal | 1 |
| Breast | 1 |
| Gall-bladder | 1 |
| Inflammatory myofibroblastic | 1 |
| Liver | 1 |
| Salivary gland | 1 |
| Uterine | 1 |
| Prior treatment | |
| None | 2 |
| 1–2 chemotherapy regimens | 10 |
| >2 chemotherapy regimens | 9 |
ECOG, Eastern Cooperative Oncology Group.
dose escalation process
The first group of subjects was infused for 48 h, and the infusion rate of troxacitabine was 3 mg/m2/day (Table 2). Four subjects were treated without incidences, and the infusion was extended for the next dose level (72-h infusion, total dose 9 mg/m2). Two DLTs were observed in the first and fifth patients of the cohort, which consisted of prolonged (≥7 day) grade 4 neutropenia (two patients) and that was associated with grade 3 thrombocytopenia in one patient. The total dose was reduced to 6 mg/m2 (72-h infusion, infusion rate 2 mg/m2/day), and three patients were treated without incidences. The next dose level escalated the total dose to 9 mg/m2 (96-h infusion, infusion rate 2.25 mg/m2/day). Two DLTs were observed in the second and fifth patients of this cohort, which consisted of grade 3 pain and constipation (one patient) and prolonged (≥7 day) grade 4 neutropenia (one patient). The total dose was reduced to 7.5 mg/m2 (96-h infusion, infusion rate 1.88 mg/m2/day), and four patients were treated without incidences. Therefore, the MTD of continuous infusion troxacitabine in patients with solid tumors is 7.5 mg/m2 administered over 96 h.
Table 2.
Dose escalation scheme and DLTs
| Dose level | Duration of infusion (h) | Infusion rate (mg/m2/day) | Total dose (mg/m2) | No. of patients | No. of cycles | No. of DLTs |
|---|---|---|---|---|---|---|
| 1 | 48 | 3 | 6 | 4 | 7 | 0 |
| 2A | 72 | 3 | 9 | 5 | 9 | 2 |
| 2B | 72 | 2 | 6 | 3 | 6 | 0 |
| 3A | 96 | 2.25 | 9 | 5 | 12 | 2 |
| 3B (MTD) | 96 | 1.88 | 7.5 | 4 | 14 | 0 |
DLT, dose-limiting toxicity; MTD, maximum tolerated dose.
toxicity
Treatment was generally well tolerated and toxicity was in concordance with the expected side-effect profile of troxacitabine. The grade 3 or 4 hematological and nonhematological events thought to be possibly, probably, or certainly related to troxacitabine and experienced during cycle 1 or during all cycles of therapy are listed in Tables 3 and 4, respectively. No grade 5 toxic effects were documented.
Table 3.
Summary of grade 3 and 4 hematological toxic effects
| Toxicity | Patients | Toxicity events by dose level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 4) |
2A (n = 5) |
2B (n = 3) |
3A (n = 5) |
3B (n = 4) |
|||||||
| Hematological | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Neutropenia | 14 | 2 | 3 | 2 | 4 | 2 | 1 | ||||
| Leukopenia | 5 | 1 | 1 | 1 | 1 | 1 | |||||
| Thrombocytopenia | 3 | 2 | 1 | ||||||||
| Anemia | 3 | 1 | 1 | 1 | |||||||
| Neutropenic fever | 1 | 1 | |||||||||
Table 4.
Summary of grade 3 and 4 non-hematological toxic effects
| Toxicity | Patients | Toxicity events by dose level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 4) |
2A (n = 5) |
2B (n = 3) |
3A (n = 5) |
3B (n = 4) |
|||||||
| Non-hematological | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Rash | 2 | 1 | 1 | ||||||||
| Nausea | 1 | 1 | |||||||||
| Hand–foot syndrome | 1 | 1 | |||||||||
| Hyperbilirubinemia | 1 | 1 | |||||||||
| Neuropathy | 1 | 1 | |||||||||
| Pruritus | 1 | 1 | |||||||||
| Elevated LFT | 1 | 1 | |||||||||
| Erythema | 1 | 1 | |||||||||
| Hypocalcemia | 1 | 1 | |||||||||
LFT, liver function tests
Hematological toxicity was dose limiting and characterized by neutropenia and thrombocytopenia. In nine patients, the neutropenia reached a grade 4 during the first cycle, in three it lasted 7 days or longer, and in one it was complicated by fever. Three patients presented with grade 3 thrombocytopenia that was uncomplicated. The most common non-hematological toxic effects were nausea, rash, fatigue, HFS, hiperbilirubinemia, and diarrhea. The first three were documented in half or more of the patients, but the majority of these toxic effects were grade 1 or 2 in severity. The only toxicity where more than one grade 3 event occurred was rash with two episodes. No non-hematological grade 4 events occurred. Whereas most of the toxic effects happened during the first cycle and their frequency or intensity did not increase with subsequent treatments, HFS typically occurred during the second cycle and thereafter.
clinical efficacy
In 18 patients, the best response was PD, and three had SD. There were three patients (all in 96-h infusion, one at 2.25 mg/m2/day and two at 1.88 mg/m2/day) with unknown origin, pancreatic and biliary cancers who received seven, five and six cycles, respectively. In these three patients, the reason for discontinuing therapy was prolonged grade 2 HFS (two patients) and suspected sepsis not related to hematologic toxicity.
PK evaluation
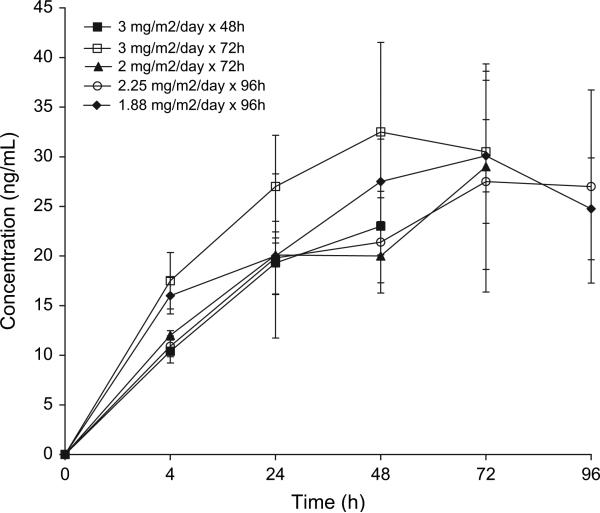
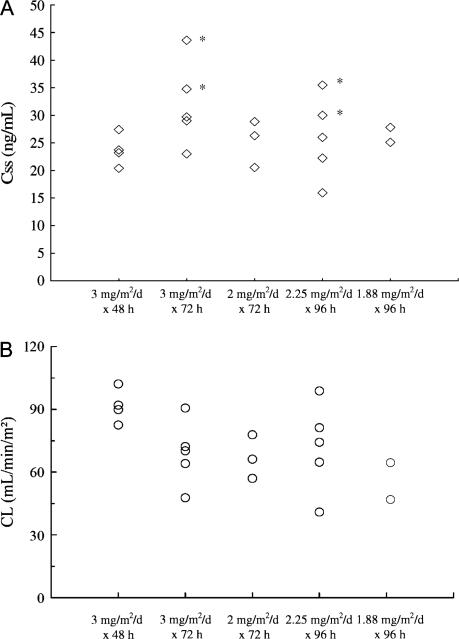
The summary PK results are shown in Table 5 and Figures 1 and 2. Of the last cohort of four patients, the analysis of two subjects was not conducted due to technical incidences. The target plasma concentration of 20 ng/ml was achieved at 24 h across all dose levels, and the levels reached a plateau after 48 h. At each time point, there were no significant differences in drug concentrations between the different infusion rate groups. Of the four patients with DLTs, three had the highest Css of all patients (Figure 2A). They also presented the lowest CL within their cohorts.
Table 5.
Summary of pharmacokinetics parameters of troxacitabine
| Parameter | 3 mg/m2/day 48 h n = 4 |
3 mg/m2/day 72 h n = 5 |
2 mg/m2/day 72 h n = 3 |
2.25 mg/m2/day 96 h n = 5 |
1.88 mg/m2/day 96 h n = 2 |
|---|---|---|---|---|---|
| Css (ng/ml) | 22.9 ± 1.99 | 31.6 ± 7.60 | 21.1 ± 3.26 | 23.7 ± 8.67 | 24.5 ± 5.4 |
| CL (ml/min/m2) | 91.6 ± 8.1 | 68.9 ± 15.4 | 67.0 ± 10.5 | 71.9 ± 21.4 | 54.3 ± 11.3 |
Values are reported as the mean ± standard deviation.
Css, steady-state plasma concentration; CL, clearance.
Figure 1.
Mean plasma concentrations of troxacitabine in the five cohorts (error bars indicate standard deviation).
Figure 2.
(A) Comparison of troxacitabine steady-state plasma concentrations (Css) across groups. Marked with asterisks are the patients with a dose-limiting toxicity. The patients (B) comparison of troxacitabine clearance (CL) across groups.
discussion
Troxacitabine is a novel cytosine analogue with an unnatural stereochemical orientation that confers on it some unique mechanistic characteristics relative to the natural stereoisomer analogue gemcitabine. Troxacitabine was initially developed in several phase I studies as multiple administrations of a short infusion, but preclinical [5] and ultimately clinical data in acute myeloid leukemia showed that continuous infusions were superior [8].
The main objective of this study was to determine whether a continuous infusion schedule achieving a presumed efficacious drug plasma level (20 ng/ml) was feasible in patients with solid tumors. The study increased infusion length in a stepwise manner, but rate of infusion had to be decreased to allow prolonged administration mainly due to hematologic toxicity. The MTD of troxacitabine given as a protracted infusion in patients with solid tumors is 1.88 mg/m2/day for 96 h, and the target concentration was achieved across dose levels after 24 h of infusion. This means that at the MTD a steady-state drug concentration of at least 20 ng/ml was maintained for 72 h. Therefore, this study successfully met its primary objectives, demonstrating that PK modeling is an efficient tool to generate hypothesis-driven studies.
Hematological toxicity was dose limiting, although only a single episode of febrile neutropenia was observed. As in previous studies, the most prevalent non-hematological toxic effects were cutaneous. These effects did not appear until the second cycle. Indeed, persistent HFS was the reason for stopping the drug in two patients who entered the study with PD and who achieved SD after five and six cycles of study drug, respectively. In terms of clinical efficacy, no responses were documented, but disease stabilization for 7, 5, and 6 months was observed in the 96-h cohorts in three patients with unknown origin, pancreatic, and biliary cancers.
As predicted, it took 24 h to reach the targeted levels and ~48 h until a plateau in Css was reached. This was particularly evident in the cohorts with a lower rate of administration. Altogether, these findings support the primary hypothesis on which this study was based and provide further rationale for the observed higher efficacy with continuous infusions. Thus, it can be inferred that only those patients receiving a 96-h infusion were above the concentration threshold for 72 h. The patients with DLTs had the highest plasma concentrations of the 21 patients in the study and also had the lowest CL within their cohorts. This is relevant and confirms the close relationship between troxacitabine PK and toxicity. The narrow rate of infusion range and a moderate interpatient variability (two-fold in terms of Css and CL within cohorts) were sufficient to obscure potential differences between cohorts in terms of Css.
In summary, troxacitabine administered by prolonged infusion in patients with solid tumors achieves drug levels that have been shown to be efficacious in preclinical models, and has a toxicity profile characterized by reversible hematological and cutaneous toxic effects. Given the poor efficacy of the short infusion trials, the hypothesis that troxacitabine as a continuous infusion will have higher antitumor efficacy can be reasonably tested in those tumor types where short troxacitabine infusions rendered negative results and for which there is solid preclinical rationale.
acknowledgements
funding
Structural Genomics.
Footnotes
Conflicts of interest: None declared.
references
- 1.Belanger K, Moore M, Baker SD, et al. Phase I and pharmacokinetic study of novel L-nucleoside analog troxacitabine given as a 30-minute infusion every 21 days. J Clin Oncol. 2002;20:2567–2574. doi: 10.1200/JCO.2002.12.047. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Stephenson J, Jr., Baker SD, et al. Troxacitabine, an L-stereoisomeric nucleoside analog, on a five-times-daily schedule: a phase I and pharmacokinetic study in patients with advanced solid malignancies. J Clin Oncol. 2002;20:96–109. doi: 10.1200/JCO.2002.20.1.96. [DOI] [PubMed] [Google Scholar]
- 3.Siu LL, Attardo G, Izbicka E, et al. Activity of (-)-2′-deoxy-3′-oxacytidine (BCH-4556) against human tumor colony-forming units. Ann Oncol. 1998;9:885–891. doi: 10.1023/a:1008387019062. [DOI] [PubMed] [Google Scholar]
- 4.Gourdeau H, Genne P, Kadhim S, et al. Antitumor activity of troxacitabine (Troxatyl) against anthracycline-resistant human xenografts. Cancer Chemother Pharmacol. 2002;50:490–496. doi: 10.1007/s00280-002-0530-7. [DOI] [PubMed] [Google Scholar]
- 5.Gourdeau H, Leblond L, Hamelin B, et al. Species differences in troxacitabine pharmacokinetics and pharmacodynamics: implications for clinical development. Clin Cancer Res. 2004;10:7692–7702. doi: 10.1158/1078-0432.CCR-04-0657. [DOI] [PubMed] [Google Scholar]
- 6.Giles FJ, Cortes JE, Baker SD, et al. Troxacitabine, a novel dioxolane nucleoside analog, has activity in patients with advanced leukemia. J Clin Oncol. 2001;19:762–771. doi: 10.1200/JCO.2001.19.3.762. [DOI] [PubMed] [Google Scholar]
- 7.Giles FJ, Garcia-Manero G, Cortes JE, et al. Phase II study of troxacitabine, a novel dioxolane nucleoside analog, in patients with refractory leukemia. J Clin Oncol. 2002;20:656–664. doi: 10.1200/JCO.2002.20.3.656. [DOI] [PubMed] [Google Scholar]
- 8.Roboz GJ, Giles FJ, Ritchie EK, et al. Phase I/II study of continuous-infusion troxacitabine in refractory acute myeloid leukemia. J Clin Oncol. 2007;25:10–15. doi: 10.1200/JCO.2006.06.6209. [DOI] [PubMed] [Google Scholar]
- 9.Townsley CA, Chi K, Ernst DS, et al. Phase II study of troxacitabine (BCH-4556) in patients with advanced and/or metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol. 2003;21:1524–1529. doi: 10.1200/JCO.2003.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Lapointe R, Letourneau R, Steward W, et al. Phase II study of troxacitabine in chemotherapy-naive patients with advanced cancer of the pancreas: gastrointestinal tumors. Ann Oncol. 2005;16:289–293. doi: 10.1093/annonc/mdi061. [DOI] [PubMed] [Google Scholar]
- 11.Dent SF, Arnold A, Stewart DJ, et al. Phase II study of troxacitabine (BCH-4556) in patients with advanced non-small-cell lung cancer. Lung. 2005;183:265–272. doi: 10.1007/s00408-004-2539-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee CK, Rowinsky EK, Li J, et al. Population pharmacokinetics of troxacitabine, a novel dioxolane nucleoside analogue. Clin Cancer Res. 2006;12:2158–2165. doi: 10.1158/1078-0432.CCR-05-2249. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]