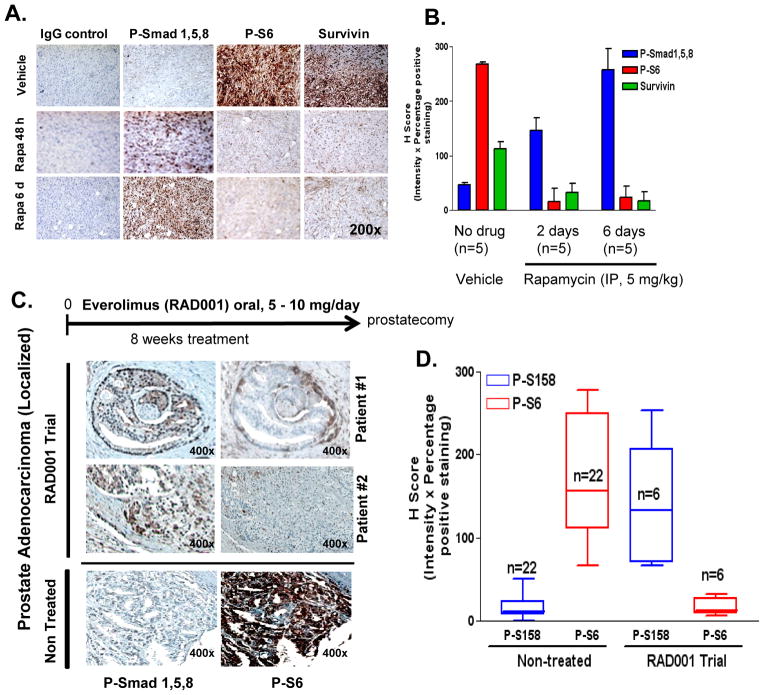
Figure 6. Rapalogs enhance P-Smad1/5/8 expression in vivo in PC3 xenografts and in tumors of patients with newly diagnosed localized prostate cancer.
A, Expression of p-Smad1/5/8, p-S6 and survivin were assessed by IHC of PC3 tumor xenografts implanted in (6–7 week old) Ncr:NU athymic male mice that received either vehicle control (n=5), or rapamycin treatment for 48 h (n=5) or 6 day (n=5) as described in methods (left); staining results were quantified by measuring H-score (% positive stained cells x staining intensity (0–3)) of matched sections (right). B, Expression of p-Smad1/5/8 and p-S6 (by IHC) in prostate tumor sections from patients with high-risk prostate cancer on a phase II clinical trial who were treated with everolimus (5 mg or 10 mg/day for 8 weeks) as neo-adjuvant therapy (n=6), and compared to non-treated control patients with prostate adenocarcinoma stage II-III (n=22) (left); H-score (% positive stained cells x staining intensity (0–3)) of non-treated control matched cores were compared to everolimus (RAD001) clinical trial matched sections (right).