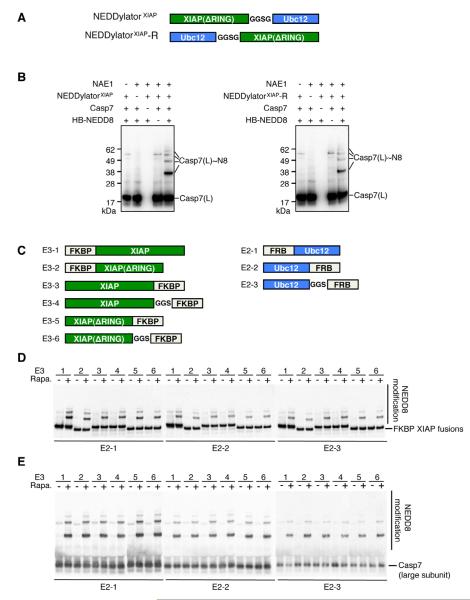
Figure 2. The NEDDylatorXIAP Activity is Dependent on Close Proximity between the XIAP Substrate Binding Domain and Ubc12, but not Restricted by the Relative Orientation between XIAP Substrate Binding domain and Ubc12.
(A) Schematic view of the NEDDylatorXIAP and NEDDylatorXIAP-R.
(B) In vitro NEDDylation of caspase-7 by the NEDDylatorXIAP and NEDDylatorXIAP-R produce similar results. Caspase-7 was NEDDylated in the presence of NEDD8 E1 (NAE1) and the NEDDylator fusions. The anti-caspase-7 antibody only recognizes the large subunit Casp-7(L). Casp-7(L)~N8 represents the NEDD8 modified form of the large subunit of caspase-7.
(C) Schematic view of the split NEDDylator, where Ubc12 is fused to FRB, and XIAP is fused to FKBP.
(D) XIAP is NEDDylated in the presence of 1 μM rapamycin with 18 combinations of split NEDDylators. Immuno-blotting was performed with an anti-XIAP antibody.
(E) Caspase-7 is NEDDylated in the presence of 1 μM rapamycin with 18 combinations of split NEDDylators. Immuno-blotting was performed with an anti-caspase-7 antibody. In (D) and (E), DMSO was added to control samples.
See Figure S1.