Fig. 6.
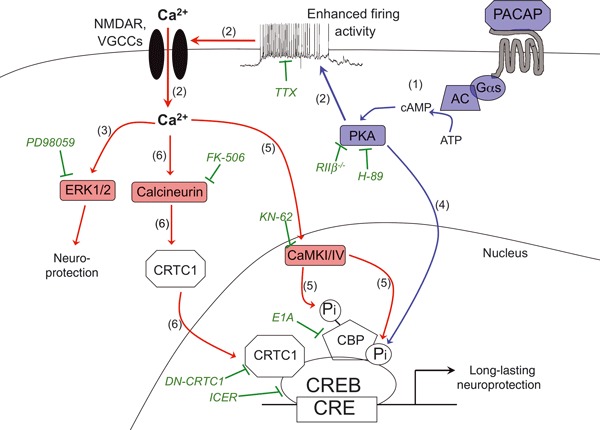
Schematic illustration of the role of activity-dependent Ca2+ signaling in PACAP-mediated neuroprotection. Activation of PACAP receptors leads to activation of PKA via the classical G-protein-adenylate cyclase (AC)-cAMP: pathway (1). PKA activation causes an increase in synaptic strength and/or neuronal excitability leading to a strong increase in levels of action potential firing which in turn triggers intracellular Ca2+ influx, likely through synaptic receptors (e.g. NMDA receptors) or voltage-gated Ca2+ channels (VGCCs): pathway (2). Activation of long-lasting neuroprotection by PACAP requires induction of gene expression mediated by the transcription factor CREB. CREB phosphorylation on serine-133 can be triggered directly by PKA in an AP firing-independent manner: pathway (3). However, this is insufficient to fully activate CREB-mediated gene expression. A key Ca2+/activity-dependent pathway involves CRTC1 nuclear translocation through activation of the Ca2+-dependent phosphatase calcineurin: pathway (4). Blue arrows and molecules indicate AP firing activity-independent events, while red arrows and molecules highlight the events dependent on AP firing. The pharmacological and genetic inhibitors of the various pathways used in this study are shown in green.
