Figure 3.
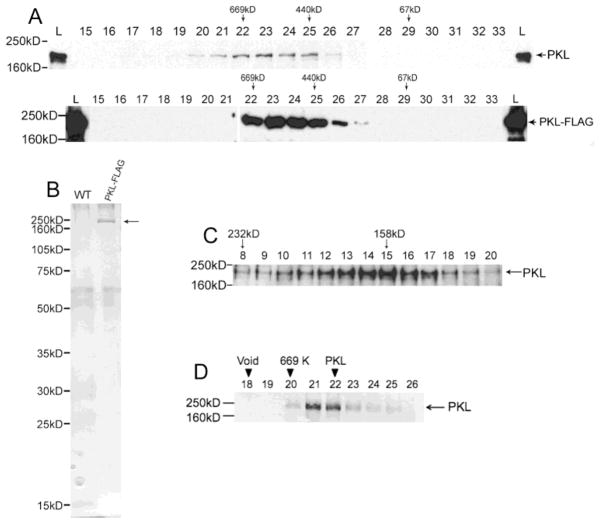
PKL exists as a monomer in plants. (A) Western analysis was used to examine fractions from a Superose 6 gel-filtration column. The westerns on top are from an extract of wild-type plants whereas the bottom westerns are from an extract of PKL-FLAG plants. A polyclonal antibody to PKL was used on the top blots, and an anti-FLAG antibody was used on the bottom blots. The fraction number is indicated by the number above each lane, L = total lysates, and MW standards for column (top) and western (left side) are indicated. PKL has been previously noted to migrate on an SDS-PAGE gel at a MW that is larger than predicted [17]. (B) Total protein from WT and PKL-FLAG plants was first passed over an anti-FLAG affinity resin and then subsequently fractionated on a sizing column as described in panel A. Fractions corresponding to lanes 22–26 of panel A were then separated on an SDS-PAGE gel and analyzed by silver staining. The arrow to right of the gel indicates PKL-FLAG protein. (C) Western analysis of fractions from a sucrose gradient using polyclonal anti-PKL antibodies. Crude cell extracts were prepared in buffer containing 350 mM NaCl. MW standards for the gradient (top) and western (left side) are indicated. (D) Recombinant PKL-FLAG protein elutes with a peak of about 650 KDa. Western analysis was used to examine fractions from a Superose 6 gel-filtration column. The fraction number is indicated by the number above each lane, L = total lysates, and MW standards for column (top) and western (left side) are indicated. The migration of the 440 kDa marker is in lanes 27–28 (not included on this blot).