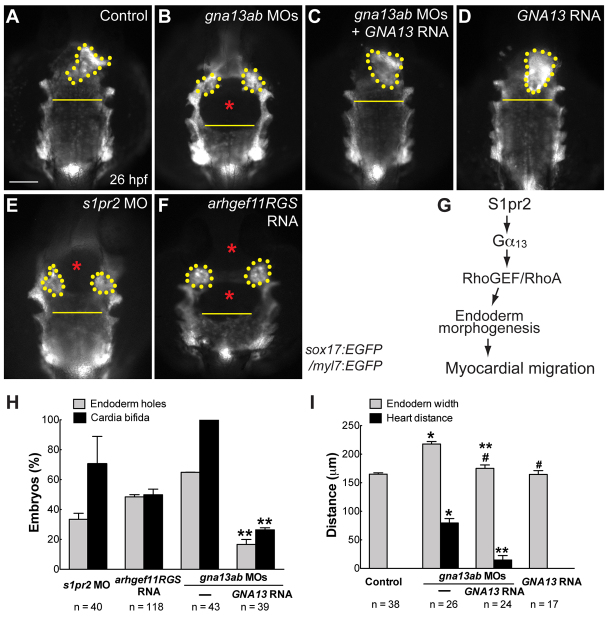
Fig. 7.
Disrupting the S1pr2/Gα13/RhoGEF signaling pathway causes defects in endoderm morphogenesis, as well as in myocardial migration. (A-F) Epifluorescence images of the anterior regions of the endoderm in 26 hpf, double-transgenic Tg(sox17:EGFP)/(myl7:EGFP) embryos. (A) Control uninjected siblings. (B-F) Embryos injected with: the gnb13ab MOs alone (B); the gnb13ab MOs plus the GNA13 RNA (C); the GNA13 RNA alone (D); the s1pr2 MO (E); and the Arhgef11RGS RNA (F). Dorsoanterior views with anterior upwards; yellow dots indicate cardiomyocytes; yellow lines (same length) indicate the width of the anterior endodermal sheet; blue asterisks indicate endodermal holes. (G) A proposed model for how S1pr2 regulates myocardial migration (see text for details). (H) Frequencies of incidences of endodermal holes and cardia bifida in 26 hpf embryos injected with the indicated MOs and RNAs. (I) Endodermal width and the distance between two populations of cardiomyocytes in 26 hpf embryos (obtained from the same pair of fish) injected with the indicated MOs and RNAs. *P< 0.01 versus control; **P<0.01 versus gna13ab MOs; #P>0.05 versus control. Data are mean±s.e.m. Scale bar: 100 μm.